Understanding immunology via engineering design: the role of mathematical prototyping
- PMID: 22973412
- PMCID: PMC3438878
- DOI: 10.1155/2012/676015
Understanding immunology via engineering design: the role of mathematical prototyping
Abstract
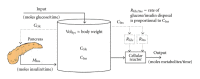
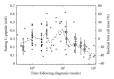
A major challenge in immunology is how to translate data into knowledge given the inherent complexity and dynamics of human physiology. Both the physiology and engineering communities have rich histories in applying computational approaches to translate data obtained from complex systems into knowledge of system behavior. However, there are some differences in how disciplines approach problems. By referring to mathematical models as mathematical prototypes, we aim to highlight aspects related to the process (i.e., prototyping) rather than the product (i.e., the model). The objective of this paper is to review how two related engineering concepts, specifically prototyping and "fitness for use," can be applied to overcome the pressing challenge in translating data into improved knowledge of basic immunology that can be used to improve therapies for disease. These concepts are illustrated using two immunology-related examples. The prototypes presented focus on the beta cell mass at the onset of type 1 diabetes and the dynamics of dendritic cells in the lung. This paper is intended to illustrate some of the nuances associated with applying mathematical modeling to improve understanding of the dynamics of disease progression in humans.
Figures




References
-
- Abbas AK, Janeway CA., Jr. Immunology: improving on nature in the twenty-first century. Cell. 2000;100(1):129–138. - PubMed
-
- FDA. Innovation or stagnation: challenge and opportunity on the critical path to new medical products. 2004.
-
- Searls DB. Data integration: challenges for drug discovery. Nature Reviews Drug Discovery. 2005;4(1):45–58. - PubMed
-
- Sorger PK, Allerheiligen SRB. Quantitative and systems pharmacology in the postgenomic era: new approaches to discovering drugs and understanding therapeutic mechanisms. 2011 An NIH white paper by the QSP workshop group, NIGMS.
-
- The Congress of the United States and Congressional Budget Office. Research and development in the pharmaceutical industry, 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

