Multiple sclerosis and pregnancy: experience from a nationwide database in Germany
- PMID: 22973421
- PMCID: PMC3437527
- DOI: 10.1177/1756285612453192
Multiple sclerosis and pregnancy: experience from a nationwide database in Germany
Abstract
Objective: The objective of this study was to evaluate exposure to disease-modifying therapies (DMTs) during pregnancy in 335 pregnancies of multiple sclerosis (MS) patients and to further determine whether exclusive breastfeeding of MS mothers has any relevant influence on postpartum relapse rate.
Background: Only limited data are available on whether DMT exposure during pregnancy affects relapse rate during pregnancy or after birth. Currently, findings on beneficial effect of exclusive breastfeeding on MS disease course are controversially discussed.
Methods: We enrolled pregnant women with MS who contacted us directly or via their treating physicians to be included in our nationwide MS and pregnancy database.
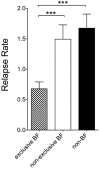
Results: We identified 78 pregnancies under interferon-beta (IFNβ) preparations, 41 under glatiramer acetate (GLAT), and 216 pregnancies without DMT exposure during pregnancy. As expected, annualized relapse rate (ARR) decreased continuously during pregnancy in nonexposed mothers (p < 0.001) to then increase after birth. In IFNβ- or GLAT-exposed women this typical pattern was not as obvious. Congenital anomalies were within normal ranges in exposed pregnancies. In total, 170 women were identified who exclusively breastfed (EBF). Significantly reduced postpartum relapse rate during the first 3 months after birth were registered in the EBF group as compared with nonexclusively breastfeeding (NEBF) or nonbreastfeeding women (NBF) women with MS (p < 0.0001). Relapse rate (RR) in the year before pregnancy had been similar throughout all groups. We did not observe any significant differences in RR of NEBF and NBF women.
Conclusion: Exclusive breastfeeding showed some beneficial effects on postpartum relapse rate in our cohort. Our data support that IFNβ and GLAT do not seem to represent a major teratogenic risk in pregnancy.
Keywords: breastfeeding; disease-modifying therapy (DMT); relapse rate.
Conflict of interest statement
Figures
References
-
- Airas L., Jalkanen A., Alanen A., Pirttila T., Marttila R. (2010) Breast-feeding, postpartum and prepregnancy disease activity in multiple sclerosis. Neurology 75: 474–476 - PubMed
-
- Amato M., Portaccio E., Ghezzi A., Hakiki B., Zipoli V., Martinelli V., et al. (2010) Pregnancy and fetal outcomes after interferon-beta exposure in multiple sclerosis. Neurology 75: 1794–1802 - PubMed
-
- Boskovic R., Wide R., Wolpin J., Bauer D., Koren G. (2005) The reproductive effects of beta interferon therapy in pregnancy: a longitudinal cohort. Neurology 65: 807–811 - PubMed
-
- Comi G., Filippi M., Wolinsky J. (2001) European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging-measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group; Ann Neurol 49: 290–297 - PubMed
-
- Confavreux C., Hutchinson M., Hours M., Cortinovis-Tourniaire P., Moreau T. (1998) Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group; N Engl J Med 339: 285–291 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

