Galectin-8 promotes cytoskeletal rearrangement in trabecular meshwork cells through activation of Rho signaling
- PMID: 22973445
- PMCID: PMC3433423
- DOI: 10.1371/journal.pone.0044400
Galectin-8 promotes cytoskeletal rearrangement in trabecular meshwork cells through activation of Rho signaling
Abstract
Purpose: The trabecular meshwork (TM) cell-matrix interactions and factors that influence Rho signaling in TM cells are thought to play a pivotal role in the regulation of aqueous outflow. The current study was designed to evaluate the role of a carbohydrate-binding protein, galectin-8 (Gal8), in TM cell adhesion and Rho signaling.
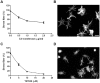
Methods: Normal human TM cells were assayed for Gal8 expression by immunohistochemistry and Western blot analysis. To assess the role of Gal8 in TM cell adhesion and Rho signaling, the cell adhesion and spreading assays were performed on Gal8-coated culture plates in the presence and the absence of anti-β₁ integrin antibody and Rho and Rho-kinase inhibitors. In addition, the effect of Gal8-mediated cell-matrix interactions on TM cell cytoskeleton arrangement and myosin light chain 2 (MLC2) phosphorylation was examined.
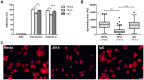
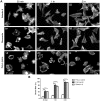
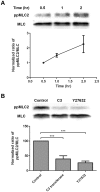
Principal findings: We demonstrate here that Gal8 is expressed in the TM and a function-blocking anti-β₁ integrin antibody inhibits the adhesion and spreading of TM cells to Gal8-coated wells. Cell spreading on Gal8 substratum was associated with the accumulation of phosphorylated myosin light chain and the formation of stress fibers that was inhibited by the Rho inhibitor, C3 transferase, as well as by the Rho-kinase inhibitor, Y27632.
Conclusions/significance: The above findings present a novel function for Gal8 in activating Rho signaling in TM cells. This function may allow Gal8 to participate in the regulation of aqueous outflow.
Conflict of interest statement
Figures

References
-
- Weinreb RN, Khaw PT (2004) Primary open-angle glaucoma. Lancet 363: 1711–1720. - PubMed
-
- Vittitow JL, Garg R, Rowlette LL, Epstein DL, O'Brien ET, et al. (2002) Gene transfer of dominant-negative RhoA increases outflow facility in perfused human anterior segment cultures. Mol Vis 8: 32–44. - PubMed
-
- Rao PV, Deng P, Maddala R, Epstein DL, Li CY, et al. (2005) Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol Vis 11: 288–297. - PubMed
-
- Polansky J, Alvarado J (1994) Cellular mechanisms influencing the aqueous humor outflow pathway. In: DM A, FA J, editors. Principles and practice of ophthalmology: basic science. Philadelphia: W. B. Saunders Co. 226–251.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

