Diversity in recognition of glycans by F-type lectins and galectins: molecular, structural, and biophysical aspects
- PMID: 22973821
- PMCID: PMC3683447
- DOI: 10.1111/j.1749-6632.2012.06698.x
Diversity in recognition of glycans by F-type lectins and galectins: molecular, structural, and biophysical aspects
Abstract
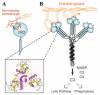
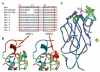
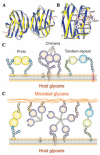
Although lectins are "hard-wired" in the germline, the presence of tandemly arrayed carbohydrate recognition domains (CRDs), of chimeric structures displaying distinct CRDs, of polymorphic genes resulting in multiple isoforms, and in some cases, of a considerable recognition plasticity of their carbohydrate binding sites, significantly expand the lectin ligand-recognition spectrum and lectin functional diversification. Analysis of structural/functional aspects of galectins and F-lectins-the most recently identified lectin family characterized by a unique CRD sequence motif (a distinctive structural fold) and nominal specificity for l-Fuc-has led to a greater understanding of self/nonself recognition by proteins with tandemly arrayed CRDs. For lectins with a single CRD, however, recognition of self and nonself glycans can only be rationalized in terms of protein oligomerization and ligand clustering and presentation. Spatial and temporal changes in lectin expression, secretion, and local concentrations in extracellular microenvironments, as well as structural diversity and spatial display of their carbohydrate ligands on the host or microbial cell surface, are suggestive of a dynamic interplay of their recognition and effector functions in development and immunity.
© 2012 New York Academy of Sciences.
Figures
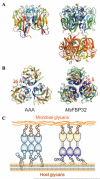
Similar articles
-
F-Type Lectins: Structure, Function, and Evolution.Methods Mol Biol. 2020;2132:225-239. doi: 10.1007/978-1-0716-0430-4_23. Methods Mol Biol. 2020. PMID: 32306331
-
Characterization of a binary tandem domain F-type lectin from striped bass (Morone saxatilis).J Biol Chem. 2006 Jan 20;281(3):1698-713. doi: 10.1074/jbc.M507652200. Epub 2005 Oct 26. J Biol Chem. 2006. PMID: 16251191
-
Structure and specificity of a binary tandem domain F-lectin from striped bass (Morone saxatilis).J Mol Biol. 2010 Aug 13;401(2):239-52. doi: 10.1016/j.jmb.2010.06.018. Epub 2010 Jun 16. J Mol Biol. 2010. PMID: 20561530 Free PMC article.
-
Lectins: getting familiar with translators of the sugar code.Molecules. 2015 Jan 22;20(2):1788-823. doi: 10.3390/molecules20021788. Molecules. 2015. PMID: 25621423 Free PMC article. Review.
-
Glycan Chains of Gangliosides: Functional Ligands for Tissue Lectins (Siglecs/Galectins).Prog Mol Biol Transl Sci. 2018;156:289-324. doi: 10.1016/bs.pmbts.2017.12.004. Epub 2018 Mar 28. Prog Mol Biol Transl Sci. 2018. PMID: 29747818 Review.
Cited by
-
Harnessing the power of mollusc lectins as immuno-protective biomolecules.Mol Biol Rep. 2024 Jan 23;51(1):182. doi: 10.1007/s11033-023-09018-8. Mol Biol Rep. 2024. PMID: 38261113 Review.
-
Galectin CvGal2 from the Eastern Oyster (Crassostrea virginica) Displays Unique Specificity for ABH Blood Group Oligosaccharides and Differentially Recognizes Sympatric Perkinsus Species.Biochemistry. 2015 Aug 4;54(30):4711-30. doi: 10.1021/acs.biochem.5b00362. Epub 2015 Jul 24. Biochemistry. 2015. PMID: 26158802 Free PMC article.
-
The regulatory power of glycans and their binding partners in immunity.Trends Immunol. 2013 Jun;34(6):290-8. doi: 10.1016/j.it.2013.01.006. Epub 2013 Feb 26. Trends Immunol. 2013. PMID: 23485517 Free PMC article. Review.
-
Epidermal LysM receptor ensures robust symbiotic signalling in Lotus japonicus.Elife. 2018 Jun 29;7:e33506. doi: 10.7554/eLife.33506. Elife. 2018. PMID: 29957177 Free PMC article.
-
Oncolytic H-1 Parvovirus Hijacks Galectin-1 to Enter Cancer Cells.Viruses. 2022 May 11;14(5):1018. doi: 10.3390/v14051018. Viruses. 2022. PMID: 35632759 Free PMC article.
References
-
- Schmidt O, et al. Role of adhesion in arthropod immune recognition. Annu. Rev. Entomol. 2010;55:485–504. - PubMed
-
- Zhang SM, et al. Diversification of Ig superfamily genes in an invertebrate. Science. 2004;305:251–254. - PubMed
-
- Watson FL, et al. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309:1874–1878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

