Multiple-site concerted proton-electron transfer reactions of hydrogen-bonded phenols are nonadiabatic and well described by semiclassical Marcus theory
- PMID: 22974135
- PMCID: PMC3476473
- DOI: 10.1021/ja305668h
Multiple-site concerted proton-electron transfer reactions of hydrogen-bonded phenols are nonadiabatic and well described by semiclassical Marcus theory
Abstract
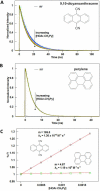
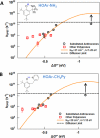
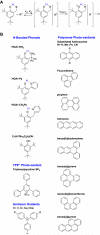
Photo-oxidations of hydrogen-bonded phenols using excited-state polyarenes are described to derive fundamental understanding of multiple-site concerted proton-electron transfer reactions (MS-CPET). Experiments have examined phenol bases having -CPh(2)NH(2), -Py, and -CH(2)Py groups ortho to the phenol hydroxyl group and tert-butyl groups in the 4,6-positions for stability (HOAr-NH(2), HOAr-Py, and HOAr-CH(2)Py, respectively; Py = pyridyl; Ph = phenyl). The photo-oxidations proceed by intramolecular proton transfer from the phenol to the pendent base concerted with electron transfer to the excited polyarene. For comparison, 2,4,6-(t)Bu(3)C(6)H(2)OH, a phenol without a pendent base and tert-butyl groups in the 2,4,6-positions, has also been examined. Many of these bimolecular reactions are fast, with rate constants near the diffusion limit. Combining the photochemical k(CPET) values with those from prior thermal stopped-flow kinetic studies gives data sets for the oxidations of HOAr-NH(2) and HOAr-CH(2)Py that span over 10(7) in k(CPET) and nearly 0.9 eV in driving force (ΔG(o)'). Plots of log(k(CPET)) vs ΔG(o)', including both excited-state anthracenes and ground state aminium radical cations, define a single Marcus parabola in each case. These two data sets are thus well described by semiclassical Marcus theory, providing a strong validation of the use of this theory for MS-CPET. The parabolas give λ(CPET) ≅ 1.15-1.2 eV and H(ab) ≅ 20-30 cm(-1). These experiments represent the most direct measurements of H(ab) for MS-CPET reactions to date. Although rate constants are available only up to the diffusion limit, the parabolas clearly peak well below the adiabatic limit of ca. 6 × 10(12) s(-1). Thus, this is a very clear demonstration that the reactions are nonadiabatic. The nonadiabatic character slows the reactions by a factor of ~45. Results for the oxidation of HOAr-Py, in which the phenol and base are conjugated, and for oxidation of 2,4,6-(t)Bu(3)C(6)H(2)OH, which lacks a base, show that both have substantially lower λ and larger pre-exponential terms. The implications of these results for MS-CPET reactions are discussed.
Figures

Similar articles
-
Concerted proton-electron transfer in the oxidation of hydrogen-bonded phenols.J Am Chem Soc. 2006 May 10;128(18):6075-88. doi: 10.1021/ja054167+. J Am Chem Soc. 2006. PMID: 16669677 Free PMC article.
-
One-electron oxidation of a hydrogen-bonded phenol occurs by concerted proton-coupled electron transfer.J Am Chem Soc. 2004 Oct 13;126(40):12718-9. doi: 10.1021/ja031583q. J Am Chem Soc. 2004. PMID: 15469234
-
Separating Proton and Electron Transfer Effects in Three-Component Concerted Proton-Coupled Electron Transfer Reactions.J Am Chem Soc. 2017 Aug 2;139(30):10312-10319. doi: 10.1021/jacs.7b03562. Epub 2017 Jul 21. J Am Chem Soc. 2017. PMID: 28671470 Free PMC article.
-
Concerted proton-electron transfers: fundamentals and recent developments.Annu Rev Anal Chem (Palo Alto Calif). 2014;7:537-60. doi: 10.1146/annurev-anchem-071213-020315. Annu Rev Anal Chem (Palo Alto Calif). 2014. PMID: 25014349 Review.
-
Proton-coupled electron transfer: a reaction chemist's view.Annu Rev Phys Chem. 2004;55:363-90. doi: 10.1146/annurev.physchem.55.091602.094446. Annu Rev Phys Chem. 2004. PMID: 15117257 Review.
Cited by
-
Roles of High-valent Hemes and pH Dependence in Halite Decomposition Catalyzed by Chlorite Dismutase from Dechloromonas aromatica.ACS Catal. 2022 Jul 15;12(14):8641-8657. doi: 10.1021/acscatal.2c01428. Epub 2022 Jul 6. ACS Catal. 2022. PMID: 35903520 Free PMC article.
-
Electron Transfer Studies of Ruthenium(II) Complexes with Biologically Important Phenolic Acids and Tyrosine.J Fluoresc. 2016 Mar;26(2):531-43. doi: 10.1007/s10895-015-1738-3. Epub 2015 Dec 8. J Fluoresc. 2016. PMID: 26645219
-
Photoinduced Electron vs. Concerted Proton Electron Transfer Pathways in SnIV (l-Tryptophanato)2 Porphyrin Conjugates.Chemistry. 2021 May 20;27(29):7872-7881. doi: 10.1002/chem.202005487. Epub 2021 May 2. Chemistry. 2021. PMID: 33780047 Free PMC article.
-
Density Functional Theory Studies on the Chemical Reactivity of Allyl Mercaptan and Its Derivatives.Molecules. 2024 Jan 31;29(3):668. doi: 10.3390/molecules29030668. Molecules. 2024. PMID: 38338412 Free PMC article.
-
Strategies for switching the mechanism of proton-coupled electron transfer reactions illustrated by mechanistic zone diagrams.Chem Sci. 2021 Dec 6;13(1):290-301. doi: 10.1039/d1sc05230f. eCollection 2021 Dec 22. Chem Sci. 2021. PMID: 35059179 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

