Epidermal growth factor receptor activation and inhibition in 3D in vitro models of normal skin and human cutaneous squamous cell carcinoma
- PMID: 22974223
- PMCID: PMC7659285
- DOI: 10.1111/cas.12026
Epidermal growth factor receptor activation and inhibition in 3D in vitro models of normal skin and human cutaneous squamous cell carcinoma
Abstract
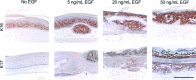
The transmembrane tyrosine kinase epidermal growth factor receptor (EGFR) is considered a key player in the development of cutaneous squamous cell carcinoma (SCC), which is the second most common malignancy in white populations. Inhibition of EGFR with the small molecule tyrosine kinase inhibitor erlotinib is currently under clinical investigation in cutaneous SCC patients. In this study, we investigated the effects of EGFR activation and inhibition on normal and malignant in vitro human skin equivalents (HSEs). In healthy HSEs, increasing EGF concentrations ranging from 5 to 50 ng/mL resulted in a dramatic decrease in epidermal proliferation as immunohistochemically assessed by Ki67 and increased epidermal stress as assessed by K17 after 2 weeks of air-exposed culture. Also, higher concentrations of EGF induced remarkable epidermal disorganization with loss of proper stratification. Similar effects were observed in HSEs generated with cutaneous SCC cell lines SCC-12B2 and SCC-13. Treatment of both healthy and SCC-HSEs with 10 μM erlotinib resulted in efficient reduction of epidermal thickness from 10 to 3 viable cell layers and counteracted EGF-induced epidermal stress. Remarkably, erlotinib treatment caused severe desquamation in healthy HSEs, reminiscent of xerosis as a known side-effect in patients treated with erlotinib. The presented three-dimensional organotypic SCC models appear suitable for further investigations on the morphological and functional impacts of modifying EGFR signaling in cutaneous SCC, without burdening patients or mice. The effective inhibition of epidermal growth by erlotinib in our HSEs confirms the therapeutic potential of this tyrosine kinase inhibitor for cutaneous SCC patients.
© 2012 Japanese Cancer Association.
Figures




Similar articles
-
Vandetanib inhibits cell growth in EGFR-expressing cutaneous squamous cell carcinoma.Biochem Biophys Res Commun. 2020 Oct 20;531(3):396-401. doi: 10.1016/j.bbrc.2020.07.111. Epub 2020 Aug 14. Biochem Biophys Res Commun. 2020. PMID: 32800552
-
Effect of erlotinib on epidermal growth factor receptor and downstream signaling in oral cavity squamous cell carcinoma.Head Neck. 2013 Sep;35(9):1323-30. doi: 10.1002/hed.23128. Epub 2012 Aug 21. Head Neck. 2013. PMID: 22907806 Free PMC article.
-
Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: molecular bases for EGFR-targeted therapy.Pathol Res Pract. 2011 Jun 15;207(6):337-42. doi: 10.1016/j.prp.2011.03.002. Epub 2011 Apr 29. Pathol Res Pract. 2011. PMID: 21531084 Review.
-
Effects of epidermal growth factor receptor and insulin-like growth factor 1 receptor inhibition on proliferation and intracellular signaling in cutaneous SCCHN: potential for dual inhibition as a therapeutic modality.Head Neck. 2013 Jan;35(1):86-93. doi: 10.1002/hed.22936. Epub 2012 Apr 12. Head Neck. 2013. PMID: 22495823 Free PMC article.
-
Epidermal Growth Factor Receptor's Function in Cutaneous Squamous Cell Carcinoma and Its Role as a Therapeutic Target in the Age of Immunotherapies.Curr Treat Options Oncol. 2020 Feb 3;21(1):9. doi: 10.1007/s11864-019-0697-3. Curr Treat Options Oncol. 2020. PMID: 32016630 Review.
Cited by
-
Molecular profiling of cutaneous squamous cell carcinomas and actinic keratoses from organ transplant recipients.BMC Cancer. 2013 Feb 5;13:58. doi: 10.1186/1471-2407-13-58. BMC Cancer. 2013. PMID: 23379751 Free PMC article.
-
Progress and Future Prospectives in Skin-on-Chip Development with Emphasis on the use of Different Cell Types and Technical Challenges.Stem Cell Rev Rep. 2017 Jun;13(3):418-429. doi: 10.1007/s12015-017-9737-1. Stem Cell Rev Rep. 2017. PMID: 28536890 Free PMC article. Review.
-
Management of High-Risk Squamous Cell Carcinoma of the Skin.Curr Treat Options Oncol. 2016 Jul;17(7):34. doi: 10.1007/s11864-016-0408-2. Curr Treat Options Oncol. 2016. PMID: 27262708 Review.
-
Interplay between Cell-Surface Receptors and Extracellular Matrix in Skin.Biomolecules. 2020 Aug 11;10(8):1170. doi: 10.3390/biom10081170. Biomolecules. 2020. PMID: 32796709 Free PMC article. Review.
-
Novel therapies for advanced skin carcinomas.Postepy Dermatol Alergol. 2020 Oct;37(5):660-670. doi: 10.5114/ada.2020.100479. Epub 2020 Nov 7. Postepy Dermatol Alergol. 2020. PMID: 33240003 Free PMC article. Review.
References
-
- Erb P, Ji J, Wernli M, Kump E, Glaser A, Buchner SA. Role of apoptosis in basal cell and squamous cell carcinoma formation. Immunol Lett 2005; 100: 68–72. - PubMed
-
- Williams H, Svensson A, Diepgen T et al Epidemiology of skin diseases in Europe. Eur J Dermatol 2006; 16: 212–8. - PubMed
-
- Kane CL, Keehn CA, Smithberger E, Glass LF. Histopathology of cutaneous squamous cell carcinoma and its variants. Semin Cutan Med Surg 2004; 23: 54–61. - PubMed
-
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006; 6: 392–401. - PubMed
-
- Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 2003; 284: 31–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

