Dosage compensation of the sex chromosomes
- PMID: 22974302
- PMCID: PMC3767307
- DOI: 10.1146/annurev-genet-110711-155454
Dosage compensation of the sex chromosomes
Abstract
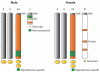
Differentiated sex chromosomes evolved because of suppressed recombination once sex became genetically controlled. In XX/XY and ZZ/ZW systems, the heterogametic sex became partially aneuploid after degeneration of the Y or W. Often, aneuploidy causes abnormal levels of gene expression throughout the entire genome. Dosage compensation mechanisms evolved to restore balanced expression of the genome. These mechanisms include upregulation of the heterogametic chromosome as well as repression in the homogametic sex. Remarkably, strategies for dosage compensation differ between species. In organisms where more is known about molecular mechanisms of dosage compensation, specific protein complexes containing noncoding RNAs are targeted to the X chromosome. In addition, the dosage-regulated chromosome often occupies a specific nuclear compartment. Some genes escape dosage compensation, potentially resulting in sex-specific differences in gene expression. This review focuses on dosage compensation in mammals, with comparisons to fruit flies, nematodes, and birds.
Figures
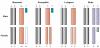

References
-
- Andina RJ. A study of X chromosome regulation during oogenesis in the mouse. Exp. Cell Res. 1978;111:211–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

