Aspirin triggered-lipoxin A4 reduces the adhesion of human polymorphonuclear neutrophils to endothelial cells initiated by preeclamptic plasma
- PMID: 22974760
- PMCID: PMC3495183
- DOI: 10.1016/j.plefa.2012.08.003
Aspirin triggered-lipoxin A4 reduces the adhesion of human polymorphonuclear neutrophils to endothelial cells initiated by preeclamptic plasma
Abstract
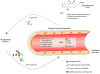
Introduction: Preeclampsia is a disorder of pregnancy, characterized by hypertension and proteinuria after 20 weeks of gestation. Here, we evaluated the role of aspirin triggered-lipoxin A(4) (ATL, 15-epi-LXA(4)) on the modulation of the adhesion of human polymorphonuclear neutrophils (PMN) to endothelial cells initiated by preeclamptic plasma.
Materials and methods: Plasma from preeclamptic, normotensive pregnant, and non-pregnant women were analyzed for factors involved in regulating angiogenesis, inflammation and lipid peroxidation. Plasma from preeclamptic women was added to human umbilical vein endothelial cells, and the adhesion of PMN (incubated with or without ATL) to cells was evaluated.
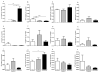
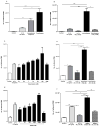
Results: Preeclampsia was associated with some augmented anti-angiogenic, oxidative and pro-inflammatory markers, as well as increasing human PMN-endothelial cell adhesion. This cell adhesion was reduced when human PMN were incubated with ATL prior to addition to endothelial monolayers.
Discussions and conclusions: Our results are the starting point for further research on the efficacy and rational use of aspirin in preeclampsia.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
C.N.S. is the inventor on patents for lipoxin analogs (aspirin triggered-lipoxin A4) that are assigned to Brigham and Women’s Hospital and licensed for clinical development. CNS’ interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Figures

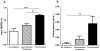

Similar articles
-
Role of aspirin-triggered lipoxin A4, aspirin, and salicylic acid in the modulation of the oxidative and inflammatory responses induced by plasma from women with pre-eclampsia.Am J Reprod Immunol. 2020 Feb;83(2):e13207. doi: 10.1111/aji.13207. Epub 2019 Nov 26. Am J Reprod Immunol. 2020. PMID: 31696583
-
The 15-Epilipoxin-A4 Pathway with Prophylactic Aspirin in Preventing Preeclampsia: A Longitudinal Cohort Study.J Clin Endocrinol Metab. 2020 Dec 1;105(12):dgaa642. doi: 10.1210/clinem/dgaa642. J Clin Endocrinol Metab. 2020. PMID: 32930782
-
Evidence that 5-lipoxygenase and acetylated cyclooxygenase 2-derived eicosanoids regulate leukocyte-endothelial adherence in response to aspirin.Br J Pharmacol. 2003 Aug;139(7):1351-9. doi: 10.1038/sj.bjp.0705356. Br J Pharmacol. 2003. PMID: 12890715 Free PMC article.
-
Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 modulate adhesion molecule expression on human leukocytes in whole blood and inhibit neutrophil-endothelial cell adhesion.Adv Exp Med Biol. 2002;507:223-8. doi: 10.1007/978-1-4615-0193-0_34. Adv Exp Med Biol. 2002. PMID: 12664589 Review. No abstract available.
-
Lipoxins and aspirin-triggered lipoxins in neutrophil adhesion and signal transduction.Prostaglandins Leukot Essent Fatty Acids. 2005 Sep-Oct;73(3-4):257-62. doi: 10.1016/j.plefa.2005.05.014. Prostaglandins Leukot Essent Fatty Acids. 2005. PMID: 15979865 Review.
Cited by
-
Molecular Targets of Aspirin and Prevention of Preeclampsia and Their Potential Association with Circulating Extracellular Vesicles during Pregnancy.Int J Mol Sci. 2019 Sep 5;20(18):4370. doi: 10.3390/ijms20184370. Int J Mol Sci. 2019. PMID: 31492014 Free PMC article. Review.
-
Role of Aspirin in High Pulsatility Index of Uterine Artery: A Consort Study.J Obstet Gynaecol India. 2018 Oct;68(5):382-388. doi: 10.1007/s13224-017-1058-4. Epub 2017 Nov 7. J Obstet Gynaecol India. 2018. PMID: 30224843 Free PMC article.
-
Specialized pro-resolving mediators: key regulators in placental function and pregnancy complications.J Mol Med (Berl). 2025 Aug;103(8):885-897. doi: 10.1007/s00109-025-02561-w. Epub 2025 Jun 7. J Mol Med (Berl). 2025. PMID: 40483361 Free PMC article. Review.
-
Aspirin: The Mechanism of Action Revisited in the Context of Pregnancy Complications.Front Immunol. 2017 Mar 15;8:261. doi: 10.3389/fimmu.2017.00261. eCollection 2017. Front Immunol. 2017. PMID: 28360907 Free PMC article. Review.
-
Targeting Angiogenesis via Resolution of Inflammation.Cold Spring Harb Perspect Med. 2023 Mar 1;13(3):a041172. doi: 10.1101/cshperspect.a041172. Cold Spring Harb Perspect Med. 2023. PMID: 35817542 Free PMC article. Review.
References
-
- Meis PJ, Goldenberg RL, Mercer BM, et al. The preterm prediction study: risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. Am J Obstet Gynecol. 1998;178:562–7. - PubMed
-
- Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–9. - PubMed
-
- Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–6. - PubMed
-
- Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens Pregnancy. 2002;21:205–23. - PubMed
-
- Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

