Acoustic radiation force for vascular cell therapy: in vitro validation
- PMID: 22975034
- PMCID: PMC3471247
- DOI: 10.1016/j.ultrasmedbio.2012.07.019
Acoustic radiation force for vascular cell therapy: in vitro validation
Abstract
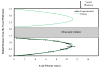
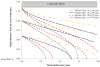
Cell-based therapeutic approaches are attractive for the restoration of the protective endothelial layer in arteries affected by atherosclerosis or following angioplasty and stenting. We have recently demonstrated a novel technique for the delivery of mesenchymal stem cells (MSCs) that are surface-coated with cationic lipid microbubbles (MBs) and displaced by acoustic radiation force (ARF) to a site of arterial injury. The objective of this study was to characterize ultrasound parameters for effective acoustic-based delivery of cell therapy. In vitro experiments were performed in a vascular flow phantom where MB-tagged MSCs were delivered toward the phantom wall using ARF generated with an intravascular ultrasound catheter. The translation motion velocity and adhesion of the MB-cell complexes were analyzed. Experimental data indicated that MSC radial velocity and adhesion to the vessel phantom increased with the time-averaged ultrasound intensity up to 1.65 W/cm², after which no further significant adhesion was observed. Temperature increase from baseline near the catheter was 5.5 ± 0.8°C with this setting. Using higher time-averaged ultrasound intensities may not significantly benefit the adhesion of MB-cell complexes to the target vessel wall (p = NS), but could cause undesirable biologic effects such as heating to the MB-cell complexes and surrounding tissue. For the highest time-averaged ultrasound intensity of 6.60 W/cm², the temperature increase was 11.6 ± 1.3°C.
Copyright © 2012 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Figures


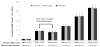



References
-
- Bechmann M, Thiel MJ, Neubauer AS, Ullrich S, Ludwig K, Kenyon KR, Ulbig MW. Central corneal thickness measurement with a retinal optical coherence tomography device versus standard ultrasonic pachymetry. Cornea. 2001;20:50–54. - PubMed
-
- Behrendt D, Ganz P. Endothelial function: From vascular biology to clinical applications. Am J Cardiol. 2002;90(10C):40L–48L. - PubMed
-
- Chen X, Apfel RE. Radiation force on a spherical object in an axisymmetric wave field and its application to the calibration of high-frequency transducers. J Acoust Soc Am. 1996;99(2):713–724. - PubMed
-
- Crum L. Bjerknes forces on bubbles in a stationary sound field. J Acoust Soc Am. 1975;57:1363–1370.
-
- Dayton PA, Morgan KE, Klibanov A, Brandenburger G, Nightingale K, Ferrara KW. A preliminary evaluation of the effects of primary and secondary radiation forces on acoustic contrast agents. IEEE Trans Ultrason Ferroelec Freq Contr. 1997;44:1264–1277.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

