Pemetrexed alters folate phenotype and inflammatory profile in EA.hy 926 cells grown under low-folate conditions
- PMID: 22975265
- PMCID: PMC4051202
- DOI: 10.1016/j.ejphar.2012.08.008
Pemetrexed alters folate phenotype and inflammatory profile in EA.hy 926 cells grown under low-folate conditions
Abstract
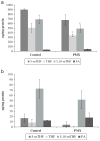
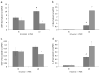
Elevated homocysteine is a risk marker for several major human pathologies. Emerging evidence suggests that perturbations of folate/homocysteine metabolism can directly modify production of inflammatory mediators. Pemetrexed acts by inhibiting thymidylate synthetase (TYMS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT). EA.hy 926 cells grown under low ("Lo") and high ("Hi") folate conditions were treated with pemetrexed. The concentrations of several intracellular folate derivatives were measured using LC-MRM/MS. Lo cells had lower total folate concentrations and a different distribution of the intracellular folate derivatives than Hi cells. Treatment with pemetrexed caused a decrease in individual folate analytes. Microarray analysis showed that several genes were significantly up or down-regulated in pemetrexed treated Lo cells. Several of the significantly up-regulated transcripts were inflammatory. Changes in transcript levels of selected targets, including C3, IL-8, and DHFR, were confirmed by quantitative RT-PCR. C3 and IL-8 transcript levels were increased in pemetrexed-treated Lo cells relative to Lo controls; DHFR transcript levels were decreased. In Lo cells, IL-8 and C3 protein concentrations were increased following pemetrexed treatment. Pemetrexed drug treatment was shown in this study to have effects that lead to an increase in pro-inflammatory mediators in Lo cells. No such changes were observed in Hi cells, suggesting that pemetrexed could not modify the inflammatory profile in the context of cellular folate sufficiency.
Copyright © 2012. Published by Elsevier B.V.
Figures

References
-
- Brown KS, Huang Y, Lu ZY, Jian W, Blair IA, Whitehead AS. Mild folate deficiency induces a proatherosclerotic phenotype in endothelial cells. Atherosclerosis. 2006;189:133–141. http://dx.doi.org/10.1016/j.atherosclerosis.2005.12.018. - DOI - PubMed
-
- Hammons AL, Summers CM, Woodside JV, McNulty H, Strain JJ, Young IS, Murray L, Boreham CA, Scott JM, Mitchell LE, Whitehead AS. Folate/homocysteine phenotypes and MTHFR 677C>T genotypes are associated with serum levels of monocyte chemoattractant protein-1. Clin Immunol. 2009;133:132–137. http://dx.doi.org/10.1016/j.clim.2009.06.008. - DOI - PMC - PubMed
-
- Huang Y, Khartulyari S, Morales ME, Stanislawska-Sachadyn A, Von Feldt JM, Whitehead AS, Blair IA. Quantification of key red blood cell folates from subjects with defined MTHFR 677C>T genotypes using stable isotope dilution liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:2403–2412. http://dx.doi.org/10.1002/rcm.3624. - DOI - PMC - PubMed
-
- Lu ZY, Jensen LE, Huang Y, Kealey C, Blair IA, Whitehead AS. The up-regulation of monocyte chemoattractant protein-1 (MCP-1) in Ea.hy 926 endothelial cells under long-term low folate stress is mediated by the p38 MAPK pathway. Atherosclerosis. 2009;205:48–54. http://dx.doi.org/10.1016/j.atherosclerosis.2008.12.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

