Lipid sorting by ceramide structure from plasma membrane to ER for the cholera toxin receptor ganglioside GM1
- PMID: 22975326
- PMCID: PMC3443397
- DOI: 10.1016/j.devcel.2012.08.002
Lipid sorting by ceramide structure from plasma membrane to ER for the cholera toxin receptor ganglioside GM1
Abstract
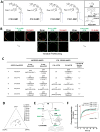
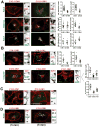
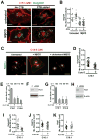
The glycosphingolipid GM1 binds cholera toxin (CT) on host cells and carries it retrograde from the plasma membrane (PM) through endosomes, the trans-Golgi (TGN), and the endoplasmic reticulum (ER) to induce toxicity. To elucidate how a membrane lipid can specify trafficking in these pathways, we synthesized GM1 isoforms with alternate ceramide domains and imaged their trafficking in live cells. Only GM1 with unsaturated acyl chains sorted efficiently from PM to TGN and ER. Toxin binding, which effectively crosslinks GM1 lipids, was dispensable, but membrane cholesterol and the lipid raft-associated proteins actin and flotillin were required. The results implicate a protein-dependent mechanism of lipid sorting by ceramide structure and provide a molecular explanation for the diversity and specificity of retrograde trafficking by CT in host cells.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no conflict of interests.
Figures




References
-
- Arab S, Lingwood CA. Influence of phospholipid chain length on verotoxin/globotriaosyl ceramide binding in model membranes: comparison of a supported b film and liposomes. Glycoconj J. 1996;13:159–166. - PubMed
-
- Badizadegan K, Wheeler HE, Fujinaga Y, Lencer WI. Trafficking of cholera toxin-ganglioside GM1 complex into Golgi and induction of toxicity depend on actin cytoskeleton. Am J Physiol Cell Physiol. 2004;287:C1453–1462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA082395/CA/NCI NIH HHS/United States
- R01 DK084424/DK/NIDDK NIH HHS/United States
- R01 DK48106/DK/NIDDK NIH HHS/United States
- P41 RR10888/RR/NCRR NIH HHS/United States
- R01 DK048106/DK/NIDDK NIH HHS/United States
- R03 DK084090/DK/NIDDK NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- R21 DK090603/DK/NIDDK NIH HHS/United States
- R01 GM097552/GM/NIGMS NIH HHS/United States
- R21 AI073409/AI/NIAID NIH HHS/United States
- P30 DK34854/DK/NIDDK NIH HHS/United States
- K01 DK073480/DK/NIDDK NIH HHS/United States
- P41 GM104603/GM/NIGMS NIH HHS/United States
- R01 CA 082395/CA/NCI NIH HHS/United States
- P41 RR010888/RR/NCRR NIH HHS/United States
- R01 GM073846/GM/NIGMS NIH HHS/United States
- R37 DK048106/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

