Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development
- PMID: 22975328
- PMCID: PMC3443394
- DOI: 10.1016/j.devcel.2012.07.015
Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development
Abstract
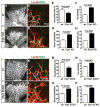
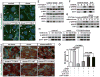
During angiogenesis, nascent vascular sprouts fuse to form vascular networks, enabling efficient circulation. Mechanisms that stabilize the vascular plexus are not well understood. Sphingosine 1-phosphate (S1P) is a blood-borne lipid mediator implicated in the regulation of vascular and immune systems. Here we describe a mechanism by which the G protein-coupled S1P receptor-1 (S1P1) stabilizes the primary vascular network. A gradient of S1P1 expression from the mature regions of the vascular network to the growing vascular front was observed. In the absence of endothelial S1P1, adherens junctions are destabilized, barrier function is breached, and flow is perturbed, resulting in abnormal vascular hypersprouting. Interestingly, S1P1 responds to S1P as well as laminar shear stress to transduce flow-mediated signaling in endothelial cells both in vitro and in vivo. These data demonstrate that blood flow and circulating S1P activate endothelial S1P1 to stabilize blood vessels in development and homeostasis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Comment in
-
S1P1 bridges mechanotransduction and angiogenesis during vascular development.Dev Cell. 2012 Sep 11;23(3):451-2. doi: 10.1016/j.devcel.2012.08.012. Dev Cell. 2012. PMID: 22975318 Free PMC article.
References
-
- Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. - PubMed
-
- Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–114. - PubMed
-
- Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

