Wnt signaling regulates postembryonic hypothalamic progenitor differentiation
- PMID: 22975330
- PMCID: PMC3445042
- DOI: 10.1016/j.devcel.2012.07.012
Wnt signaling regulates postembryonic hypothalamic progenitor differentiation
Abstract
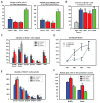
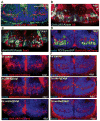
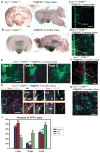
Previous studies have raised the possibility that Wnt signaling may regulate both neural progenitor maintenance and neuronal differentiation within a single population. Here we investigate the role of Wnt/β-catenin activity in the zebrafish hypothalamus and find that the pathway is first required for the proliferation of unspecified hypothalamic progenitors in the embryo. At later stages, including adulthood, sequential activation and inhibition of Wnt activity is required for the differentiation of neural progenitors and negatively regulates radial glia differentiation. The presence of Wnt activity is conserved in hypothalamic progenitors of the adult mouse, where it plays a conserved role in inhibiting the differentiation of radial glia. This study establishes the vertebrate hypothalamus as a model for Wnt-regulated postembryonic neural progenitor differentiation and defines specific roles for Wnt signaling in neurogenesis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. - PubMed
-
- Chapouton P, Jagasia R, Bally-Cuif L. Adult neurogenesis in non-mammalian vertebrates. Bioessays. 2007;29:745–757. - PubMed
-
- Chapouton P, Webb KJ, Stigloher C, Alunni A, Adolf B, Hesl B, Topp S, Kremmer E, Bally-Cuif L. Expression of hairy/enhancer of split genes in neural progenitors and neurogenesis domains of the adult zebrafish brain. The Journal of comparative neurology. 2011;519:1748–1769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

