EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma
- PMID: 22975375
- PMCID: PMC3601542
- DOI: 10.1016/j.ccr.2012.08.001
EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma
Abstract
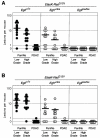
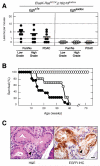
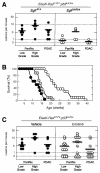
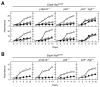
Clinical evidence indicates that mutation/activation of EGF receptors (EGFRs) is mutually exclusive with the presence of K-RAS oncogenes in lung and colon tumors. We have validated these observations using genetically engineered mouse models. However, development of pancreatic ductal adenocarcinomas driven by K-Ras oncogenes are totally dependent on EGFR signaling. Similar results were obtained using human pancreatic tumor cell lines. EGFRs were also essential even in the context of pancreatic injury and absence of p16Ink4a/p19Arf. Only loss of p53 made pancreatic tumors independent of EGFR signaling. Additional inhibition of PI3K and STAT3 effectively prevented proliferation of explants derived from these p53-defective pancreatic tumors. These findings may provide the bases for more rational approaches to treat pancreatic tumors in the clinic.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Tumorigenesis: Pushing pancreatic cancer to take off.Nat Rev Cancer. 2012 Nov;12(11):739. doi: 10.1038/nrc3383. Epub 2012 Oct 5. Nat Rev Cancer. 2012. PMID: 23037449 No abstract available.
References
-
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguría A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. Tumour biology: senescence inpremalignant tumours. Nature. 2005;436:642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

