Connexin and pannexin hemichannels in inflammatory responses of glia and neurons
- PMID: 22975435
- PMCID: PMC3627726
- DOI: 10.1016/j.brainres.2012.08.042
Connexin and pannexin hemichannels in inflammatory responses of glia and neurons
Abstract
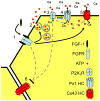
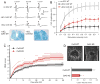
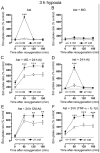
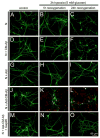
Mammals express ∼20 different connexins, the main gap junction forming proteins in mammals, and 3 pannexins, homologs of innexins, the main gap junction forming proteins in invertebrates. In both classes of gap junction, each channel is formed by two hemichannels, one contributed by each of the coupled cells. There is now general, if not universal, agreement that hemichannels of both classes can open in response to various physiological and pathological stimuli when they are not apposed to another hemichannels and face the external milieu. Connexin (and likely pannexin) hemichannel permeability is consistent with that of the cell-cell channels and open hemichannels can be a release site for relatively large molecules such as ATP and glutamate, which can serve as transmitters between cells. Here we describe three experimental paradigms in which connexin and pannexin hemichannel signaling occurs. (1) In cultures of spinal astrocytes FGF-1 causes the release of ATP, and ATP causes opening of pannexin hemichannels, which then release further ATP. Subsequently, several hours later, connexin hemichannels are also opened by an unknown mechanism. Release of ATP appears to become self sustaining through action of P2X7 receptors to open pannexin hemichannels and then connexin hemichannels, both of which are ATP permeable. (2) Spinal cord injury by dropping a small weight on the exposed cord is followed by release of ATP in the region surrounding the primary lesion. This release is greatly reduced in a mouse in which Cx43 is knocked down in the astrocytes. Application of FGF-1 causes a similar release of ATP in the uninjured spinal cord, and an inhibitor of the FGF-1 receptor, PD173074, inhibits both FGF-1 and injury-induced release. Reduction in ATP release is associated with reduced inflammation and less secondary expansion of the lesion. (3) Cortical astrocytes in culture are permeabilized by hypoxia, and this effect is increased by high or zero glucose. The mechanism of permeabilization is opening of Cx43 hemichannels, which can lead to cell death. Activated microglia secrete TNF-α and IL-1β, which open connexin hemichannels in astrocytes. Astrocytes release ATP and glutamate which can kill neurons in co-culture through activation of neuronal pannexin hemichannels. These studies implicate two kinds of gap junction hemichannel in inflammatory responses and cell death. This article is part of a Special Issue entitled Electrical Synapses.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures





References
-
- Anderson KM, Tsui P, Guinan P, Rubenstein M. The proliferative response of hela cells to 2-deoxy-D-glucose under hypoxic or anoxic conditions: An analogue for studying some properties of in vivo solid cancers. Anticancer Res. 2006;26:4155–4162. - PubMed
-
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–16. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
-
- Bondarenko A, Chesler M. Glia. 2001;34:134–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

