Electrophysiological changes precede morphological changes to frontal cortical pyramidal neurons in the rTg4510 mouse model of progressive tauopathy
- PMID: 22976049
- PMCID: PMC3509230
- DOI: 10.1007/s00401-012-1038-9
Electrophysiological changes precede morphological changes to frontal cortical pyramidal neurons in the rTg4510 mouse model of progressive tauopathy
Abstract
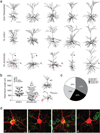
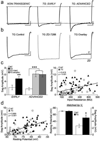
Whole-cell patch-clamp recordings and high-resolution morphometry were used to assess functional and structural properties of layer 3 pyramidal neurons in early (<4 months) and advanced (>8 months) stages of tauopathy in frontal cortical slices prepared from rTg4510 tau mutant (P301L) mice. In early tauopathy, dendritic architecture is preserved. In advanced tauopathy, neurons can be categorized as either "atrophic" (58 %)-exhibiting marked atrophy of the apical tuft, or "intact" (42 %)-with normal apical tufts and, in some instances, proliferative sprouting of oblique branches of the apical trunk. Approximately equal numbers of atrophic and intact neurons contain neurofibrillary tangles (NFTs) or are tangle-free, lending further support to the idea that NFTs per se are not toxic. Spine density is decreased due to a specific reduction in mushroom spines, but filopodia are increased in both atrophic and intact neurons. By contrast to these morphological changes, which are robust only in the advanced stage, significant electrophysiological changes are present in the early stage and persist in the advanced stage in both atrophic and intact neurons. The most marked of these changes are: a depolarized resting membrane potential, an increased depolarizing sag potential and increased action potential firing rates-all indicative of hyperexcitability. Spontaneous excitatory postsynaptic currents are not reduced in frequency or amplitude in either stage. The difference in the time course of functionally important electrophysiological changes versus regressive morphological changes implies differences in pathogenic mechanisms underlying functional and structural changes to neurons during progressive tauopathy.
Figures




References
-
- Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci U S A. 1997;94(1):298–303. - PMC - PubMed
-
- Arendt T. Alzheimer's disease as a disorder of mechanisms underlying structural brain self-organization. Neuroscience. 2001;102(4):723–765. - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42(3 Pt 1):631–639. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

