Bispecific antibody derivatives with restricted binding functionalities that are activated by proteolytic processing
- PMID: 22976197
- PMCID: PMC3449404
- DOI: 10.1093/protein/gzs064
Bispecific antibody derivatives with restricted binding functionalities that are activated by proteolytic processing
Abstract
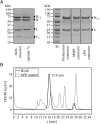
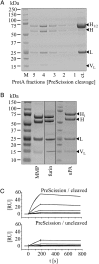
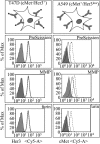
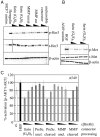
We have designed bispecific antibodies that bind one target (anti-Her3) in a bivalent IgG-like manner and contain one additional binding entity (anti-cMet) composed of one V(H) and one V(L) domain connected by a disulfide bond. The molecules are assembled by fusing a V(H,Cys44) domain via flexible connector peptides to the C-terminus of one H-chain (heavy chain), and a V(L,Cys100) to another H-chain. To ensure heterodimerization during expression in mammalian cells, we introduced complementary knobs-into-holes mutations into the different H-chains. The IgG-shaped trivalent molecules carry as third binding entity one disulfide-stabilized Fv (dsFv) without a linker between V(H) and V(L). Tethering the V(H) and V(L) domains at the C-terminus of the C(H)3 domain decreases the on-rates of the dsFv to target antigens without affecting off-rates. Steric hindrance resolves upon removal of one side of the double connection by proteolysis: this improves flexibility and accessibility of the dsFv and fully restores antigen access and affinity. This technology has multiple applications: (i) in cases where single-chain linkers are not desired, dsFvs without linkers can be generated by addition of furin site(s) in the connector that are processed during expression within mammalian cells; (ii) highly active (toxic) entities which affect expression can be produced as inactive dsFvs and subsequently be activated (e.g. via PreScission cleavage) during purification; (iii) entities can be generated which are targeted by the unrestricted binding entity and can be activated by proteases in target tissues. For example, Her3-binding molecules containing linkers with recognition sequences for matrix metalloproteases or urokinase, whose inactivated cMet binding site is activated by proteolytic processing.
Figures

Similar articles
-
"BIClonals": Production of Bispecific Antibodies in IgG Format in Transiently Transfected Mammalian Cells.Methods Mol Biol. 2019;1904:431-454. doi: 10.1007/978-1-4939-8958-4_22. Methods Mol Biol. 2019. PMID: 30539485
-
A novel glycoengineered bispecific antibody format for targeted inhibition of epidermal growth factor receptor (EGFR) and insulin-like growth factor receptor type I (IGF-1R) demonstrating unique molecular properties.J Biol Chem. 2014 Jul 4;289(27):18693-706. doi: 10.1074/jbc.M113.528109. Epub 2014 May 19. J Biol Chem. 2014. PMID: 24841203 Free PMC article.
-
A modular IgG-scFv bispecific antibody topology.Protein Eng Des Sel. 2010 Apr;23(4):221-8. doi: 10.1093/protein/gzp077. Epub 2009 Dec 17. Protein Eng Des Sel. 2010. PMID: 20019028 Free PMC article.
-
Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies.MAbs. 2012 Nov-Dec;4(6):653-63. doi: 10.4161/mabs.21379. Epub 2012 Aug 27. MAbs. 2012. PMID: 22925968 Free PMC article. Review.
-
Antibody engineering of recombinant Fv immunotoxins for improved targeting of cancer: disulfide-stabilized Fv immunotoxins.Clin Cancer Res. 1996 Feb;2(2):245-52. Clin Cancer Res. 1996. PMID: 9816166 Review.
Cited by
-
The Masking Game: Design of Activatable Antibodies and Mimetics for Selective Therapeutics and Cell Control.ACS Cent Sci. 2021 May 26;7(5):724-738. doi: 10.1021/acscentsci.0c01448. Epub 2021 Apr 26. ACS Cent Sci. 2021. PMID: 34079893 Free PMC article. Review.
-
Prodrug-based bispecific antibodies for cancer therapy: advances and future directions.Front Immunol. 2025 Jan 22;16:1523693. doi: 10.3389/fimmu.2025.1523693. eCollection 2025. Front Immunol. 2025. PMID: 39911391 Free PMC article. Review.
-
Nanocell targeting using engineered bispecific antibodies.MAbs. 2015;7(1):53-65. doi: 10.4161/19420862.2014.985952. MAbs. 2015. PMID: 25523746 Free PMC article.
-
The making of bispecific antibodies.MAbs. 2017 Feb/Mar;9(2):182-212. doi: 10.1080/19420862.2016.1268307. MAbs. 2017. PMID: 28071970 Free PMC article. Review.
-
Antibody Engineering & Therapeutics, the annual meeting of The Antibody Society December 7-10, 2015, San Diego, CA, USA.MAbs. 2016;8(3):617-52. doi: 10.1080/19420862.2016.1153211. MAbs. 2016. PMID: 26909869 Free PMC article.
References
-
- Bao W., Fu H.J., Jia L.T., et al. Arch. Biochem. Biophys. 2010;499:49–55. - PubMed
-
- Brinkmann U., Haas A.K., Metz S., Schanzer J. 2012a. (March 1) World Intellectual Property Organization Publication WO/2012/025530 (patentscope.wipo.int)
-
- Brinkmann U., Croasdale R., Metz S., Schanzer J., Sustmann C., Umana P. 2012b. (March 1) World Intellectual Property Organization Publication WO/2012/025525. (patentscope.wipo.int)
-
- Buchner J., Pastan I., Brinkmann U. Anal. Biochem. 1992;205:263–270. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

