Estrogen-induced maldevelopment of the penis involves down-regulation of myosin heavy chain 11 (MYH11) expression, a biomarker for smooth muscle cell differentiation
- PMID: 22976277
- PMCID: PMC3509779
- DOI: 10.1095/biolreprod.112.103556
Estrogen-induced maldevelopment of the penis involves down-regulation of myosin heavy chain 11 (MYH11) expression, a biomarker for smooth muscle cell differentiation
Abstract
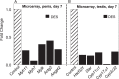
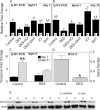
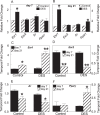
Cavernous smooth muscle cells are essential components in penile erection. In this study, we investigated effects of estrogen exposure on biomarkers for smooth muscle cell differentiation in the penis. Neonatal rats received diethylstilbestrol (DES), with or without the estrogen receptor (ESR) antagonist ICI 182,780 (ICI) or the androgen receptor (AR) agonist dihydrotestosterone (DHT), from Postnatal Days 1 to 6. Tissues were collected at 7, 10, or 21 days of age. The smooth muscle cell biomarker MYH11 was studied in depth because microarray data showed it was significantly down-regulated, along with other biomarkers, in DES treatment. Quantitative real time-PCR and Western blot analyses showed 50%-80% reduction (P ≤ 0.05) in Myh11 expression in DES-treated rats compared to that in controls; and ICI and DHT coadministration mitigated the decrease. Temporally, from 7 to 21 days of age, Myh11 expression was onefold increased (P ≥ 0.05) in DES-treated rats versus threefold increased (P ≤ 0.001) in controls, implying the long-lasting inhibitory effect of DES on smooth muscle cell differentiation. Immunohistochemical localization of smooth muscle alpha actin, another biomarker for smooth muscle cell differentiation, showed fewer cavernous smooth muscle cells in DES-treated animals than in controls. Additionally, DES treatment significantly up-regulated Esr1 mRNA expression and suppressed the neonatal testosterone surge by 90%, which was mitigated by ICI coadministration but not by DHT coadministration. Collectively, results provided evidence that DES treatment in neonatal rats inhibited cavernous smooth muscle cell differentiation, as shown by down-regulation of MYH11 expression at the mRNA and protein levels and by reduced immunohistochemical staining of smooth muscle alpha actin. Both the ESR and the AR pathways probably mediate this effect.
Figures




Similar articles
-
Low androgen induced penile maldevelopment involves altered gene expression of biomarkers of smooth muscle differentiation and a key enzyme regulating cavernous smooth muscle cell tone.J Urol. 2014 Jul;192(1):267-73. doi: 10.1016/j.juro.2013.11.101. Epub 2013 Dec 6. J Urol. 2014. PMID: 24316094
-
Estrogen-induced developmental disorders of the rat penis involve both estrogen receptor (ESR)- and androgen receptor (AR)-mediated pathways.Biol Reprod. 2009 Sep;81(3):507-16. doi: 10.1095/biolreprod.108.071951. Epub 2009 May 6. Biol Reprod. 2009. PMID: 19420389 Free PMC article.
-
Exposure of neonatal rats to anti-androgens induces penile mal-developments and infertility comparable to those induced by oestrogens.Int J Androl. 2012 Jun;35(3):364-76. doi: 10.1111/j.1365-2605.2011.01232.x. Epub 2011 Dec 13. Int J Androl. 2012. PMID: 22150386
-
Testosterone down-regulates the levels of androgen receptor mRNA in smooth muscle cells from the rat corpora cavernosa via aromatization to estrogens.J Steroid Biochem Mol Biol. 1993 May;45(5):333-43. doi: 10.1016/0960-0760(93)90002-e. J Steroid Biochem Mol Biol. 1993. PMID: 8499343
-
Role of estrogen in induction of penile dysmorphogenesis: a review.Reproduction. 2007 Aug;134(2):199-208. doi: 10.1530/REP-07-0207. Reproduction. 2007. PMID: 17660230 Review.
Cited by
-
Estradiol is an independent risk factor for organic erectile dysfunction in eugonadal young men.Asian J Androl. 2020 Nov-Dec;22(6):636-641. doi: 10.4103/aja.aja_135_19. Asian J Androl. 2020. PMID: 31929197 Free PMC article.
-
Comparative effects of neonatal diethylstilbestrol on external genitalia development in adult males of two mouse strains with differential estrogen sensitivity.Differentiation. 2014 Sep-Oct;88(2-3):70-83. doi: 10.1016/j.diff.2014.09.004. Epub 2014 Oct 16. Differentiation. 2014. PMID: 25449353 Free PMC article.
-
Exploring disease-specific methylated CpGs in human male genital abnormalities by using methylated-site display-amplified fragment length polymorphism (MSD-AFLP).J Reprod Dev. 2019 Dec 18;65(6):491-497. doi: 10.1262/jrd.2019-069. Epub 2019 Aug 29. J Reprod Dev. 2019. PMID: 31462596 Free PMC article.
-
Bisphenol A, Dichlorodiphenyltrichloroethane (DDT) and Vinclozolin Affect ex-vivo Uterine Contraction in Rats via Uterotonin (Prostaglandin F2α, Acetylcholine and Oxytocin) Related Pathways.Int J Med Sci. 2015 Oct 27;12(11):914-25. doi: 10.7150/ijms.11957. eCollection 2015. Int J Med Sci. 2015. PMID: 26640411 Free PMC article.
-
Distinct molecular subtypes of uterine leiomyosarcoma respond differently to chemotherapy treatment.BMC Cancer. 2017 Sep 11;17(1):639. doi: 10.1186/s12885-017-3568-y. BMC Cancer. 2017. PMID: 28893210 Free PMC article.
References
-
- Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res 2008; 20:17 29 - PubMed
-
- Burnett AL. Molecular pharmacotherapeutic targeting of PDE5 for preservation of penile health. J Androl 2008; 29:3 14 - PubMed
-
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 1999; 84:50 56 - PubMed
-
- Nehra A, Barrett DM, Moreland RB. Pharmacotherapeutic advances in the treatment of erectile dysfunction. Mayo Clin Proc 1999; 74:709 721 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

