RIN1 orchestrates the activation of RAB5 GTPases and ABL tyrosine kinases to determine the fate of EGFR
- PMID: 22976291
- PMCID: PMC3575715
- DOI: 10.1242/jcs.113688
RIN1 orchestrates the activation of RAB5 GTPases and ABL tyrosine kinases to determine the fate of EGFR
Abstract
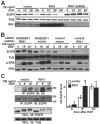
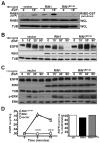
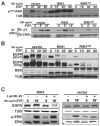
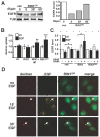
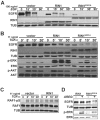
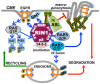
Stimulation of epidermal growth factor receptor (EGFR) initiates RAS signaling simultaneously with EGFR internalization. Endocytosed EGFR is then either recycled or degraded. EGFR fate is determined in part by the RAS effector RIN1, a guanine nucleotide exchange factor (GEF) for RAB5 GTPases. EGFR degradation was slowed by RIN1 silencing, enhanced by RIN1 overexpression and accelerated by RIN1 localization to the plasma membrane. RIN1 also directly activates ABL tyrosine kinases, which regulate actin remodeling, a function not previously connected to endocytosis. We report that RIN1-RAB5 signaling favors EGFR downregulation over EGFR recycling, whereas RIN1-ABL signaling stabilizes EGFR and inhibits macropinocytosis. RIN1(QM), a mutant that blocks ABL activation, caused EGF-stimulated membrane ruffling, actin remodeling, dextran uptake and EGFR degradation. An ABL kinase inhibitor phenocopied these effects in cells overexpressing RIN1. EGFR activation also promotes RIN1 interaction with BIN1, a membrane bending protein. These findings suggest that RIN1 orchestrates RAB5 activation, ABL kinase activation and BIN1 recruitment to determine EGFR fate.
Figures


References
-
- Andoniou C. E., Thien C. B., Langdon W. Y. (1996). The two major sites of cbl tyrosine phosphorylation in abl-transformed cells select the crkL SH2 domain. Oncogene 12, 1981–1989 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

