Intercellular adhesion molecule-1 is a regulator of blood-testis barrier function
- PMID: 22976294
- PMCID: PMC3575703
- DOI: 10.1242/jcs.107987
Intercellular adhesion molecule-1 is a regulator of blood-testis barrier function
Abstract
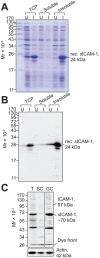
The mechanism underlying the movement of preleptotene/leptotene spermatocytes across the blood-testis barrier (BTB) during spermatogenesis is not well understood largely owing to the fact that the BTB, unlike most other blood-tissue barriers, is composed of several co-existing and co-functioning junction types. In the present study, we show that intercellular adhesion molecule-1 [ICAM-1, a Sertoli and germ cell adhesion protein having five immunoglobulin (Ig)-like domains, in addition to transmembrane and cytoplasmic domains] is a regulator of BTB integrity. Initial experiments showed ICAM-1 to co-immunoprecipitate and co-localize with tight junction and basal ectoplasmic specialization proteins such as occludin and N-cadherin, which contribute to BTB function. More importantly, overexpression of ICAM-1 in Sertoli cells in vitro enhanced barrier function when monitored by transepithelial electrical resistance measurements, illustrating that ICAM-1-mediated adhesion can promote BTB integrity. On the other hand, overexpression of a truncated form of ICAM-1 that consisted only of the five Ig-like domains (sICAM-1; this form of ICAM-1 is known to be secreted) elicited an opposite effect when Sertoli cell barrier function was found to be perturbed in vitro; in this case, sICAM-1 overexpression resulted in the downregulation of several BTB constituent proteins, which was probably mediated by Pyk2/p-Pyk2-Y402 and c-Src/p-Src-Y530. These findings were expanded to the in vivo level when BTB function was found to be disrupted following sICAM-1 overexpression. These data illustrate the existence of a unique mechanism in the mammalian testis where ICAM-1 can either positively or negatively regulate BTB function.
Figures





References
-
- Byers S. W., Hadley M. A., Djakiew D., Dym M. (1986). Growth and characterization of polarized monolayers of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J. Androl. 7, 59–68 - PubMed
-
- Cheng C. Y., Bardin C. W. (1987). Identification of two testosterone-responsive testicular proteins in Sertoli cell-enriched culture medium whose secretion is suppressed by cells of the intact seminiferous tubule. J. Biol. Chem. 262, 12768–12779 - PubMed
-
- Cheng C. Y., Mruk D. D. (2002). Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 82, 825–874 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

