The SUMO ligase PIAS1 regulates UV-induced apoptosis by recruiting Daxx to SUMOylated foci
- PMID: 22976298
- PMCID: PMC3575712
- DOI: 10.1242/jcs.110825
The SUMO ligase PIAS1 regulates UV-induced apoptosis by recruiting Daxx to SUMOylated foci
Abstract
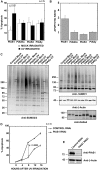
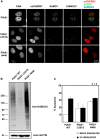
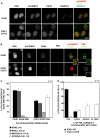
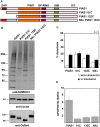
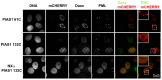
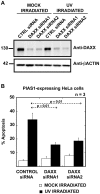
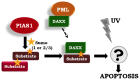
The small ubiquitin-like modifier (SUMO) ligase PIAS1 (Protein Inhibitor of Activated Stat-1) has been shown to play a role in cellular stress response by SUMOylating several proteins that are involved in DNA repair, apoptosis and transcription. In this paper, we show that PIAS1 regulates ultraviolet (UV)-induced apoptosis by recruiting Death-associated protein 6 (Daxx) to PIAS1-generated SUMO-foci. Cells that ectopically express PIAS1, but not other PIASes, show increased sensitivity to UV irradiation, suggesting that PIAS1 has a distinct function in UV-dependent apoptosis. Domain analysis of PIAS1 indicates that both PIAS1 SUMO-ligase activity and the specific localization of PIAS1 through its N-terminal and C-terminal domains are essential for UV-induced cell death. Daxx colocalizes with PIAS1-generated SUMOylated foci, and the reduction of Daxx using RNAi alleviates UV-induced apoptosis in PIAS1-expressing cells. PIAS1-mediated recruitment of Daxx and apoptosis following UV irradiation are dependent upon the Daxx C-terminal SUMO-interacting motif (SIM). Overall, our data suggest that the pro-apoptotic protein Daxx specifically interacts with one or more substrates SUMOylated by PIAS1 and this interaction leads to apoptosis following UV irradiation.
Figures

References
-
- Carpenter A. E., Jones T. R., Lamprecht M. R., Clarke C., Kang I. H., Friman O., Guertin D. A., Chang J. H., Lindquist R. A., Moffat J.et al. (2006). CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 10.1186/gb-2006-7-10-r100 - DOI - PMC - PubMed
-
- Chang C. C., Naik M. T., Huang Y. S., Jeng J. C., Liao P. H., Kuo H. Y., Ho C. C., Hsieh Y. L., Lin C. H., Huang N. J.et al. (2011). Structural and functional roles of Daxx SIM phosphorylation in SUMO paralog-selective binding and apoptosis modulation. Mol. Cell 42, 62–74 10.1016/j.molcel.2011.02.022 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

