Transcriptional regulation of Profilin during wound closure in Drosophila larvae
- PMID: 22976306
- PMCID: PMC3575702
- DOI: 10.1242/jcs.107490
Transcriptional regulation of Profilin during wound closure in Drosophila larvae
Abstract
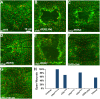
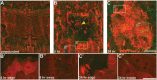
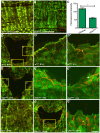
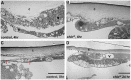
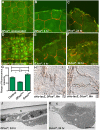
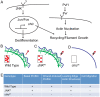
Injury is an inevitable part of life, making wound healing essential for survival. In postembryonic skin, wound closure requires that epidermal cells recognize the presence of a gap and change their behavior to migrate across it. In Drosophila larvae, wound closure requires two signaling pathways [the Jun N-terminal kinase (JNK) pathway and the Pvr receptor tyrosine kinase signaling pathway] and regulation of the actin cytoskeleton. In this and other systems, it remains unclear how the signaling pathways that initiate wound closure connect to the actin regulators that help execute wound-induced cell migrations. Here, we show that chickadee, which encodes the Drosophila Profilin, a protein important for actin filament recycling and cell migration during development, is required for the physiological process of larval epidermal wound closure. After injury, chickadee is transcriptionally upregulated in cells proximal to the wound. We found that JNK, but not Pvr, mediates the increase in chic transcription through the Jun and Fos transcription factors. Finally, we show that chic-deficient larvae fail to form a robust actin cable along the wound edge and also fail to form normal filopodial and lamellipodial extensions into the wound gap. Our results thus connect a factor that regulates actin monomer recycling to the JNK signaling pathway during wound closure. They also reveal a physiological function for an important developmental regulator of actin and begin to tease out the logic of how the wound repair response is organized.
Figures

Similar articles
-
A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes.Genetics. 2010 Nov;186(3):943-57. doi: 10.1534/genetics.110.121822. Epub 2010 Sep 2. Genetics. 2010. PMID: 20813879 Free PMC article.
-
cdc37 is essential for JNK pathway activation and wound closure in Drosophila.Mol Biol Cell. 2019 Oct 1;30(21):2651-2658. doi: 10.1091/mbc.E18-12-0822. Epub 2019 Sep 4. Mol Biol Cell. 2019. PMID: 31483695 Free PMC article.
-
A blood-borne PDGF/VEGF-like ligand initiates wound-induced epidermal cell migration in Drosophila larvae.Curr Biol. 2009 Sep 15;19(17):1473-7. doi: 10.1016/j.cub.2009.07.019. Epub 2009 Jul 30. Curr Biol. 2009. PMID: 19646875 Free PMC article.
-
Crawling wounded: molecular genetic insights into wound healing from Drosophila larvae.Int J Dev Biol. 2018;62(6-7-8):479-489. doi: 10.1387/ijdb.180085mg. Int J Dev Biol. 2018. PMID: 29938760 Free PMC article. Review.
-
Signaling pathways directing the movement and fusion of epithelial sheets: lessons from dorsal closure in Drosophila.Differentiation. 2002 Jun;70(4-5):181-203. doi: 10.1046/j.1432-0436.2002.700408.x. Differentiation. 2002. PMID: 12147138 Review.
Cited by
-
JNK signaling and integrins cooperate to maintain cell adhesion during epithelial fusion in Drosophila.Front Cell Dev Biol. 2024 Jan 9;11:1034484. doi: 10.3389/fcell.2023.1034484. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38264353 Free PMC article.
-
Casein kinase 1α decreases β-catenin levels at adherens junctions to facilitate wound closure in Drosophila larvae.Development. 2019 Oct 2;146(20):dev175133. doi: 10.1242/dev.175133. Development. 2019. PMID: 31511254 Free PMC article.
-
A scar-like lesion is apparent in basement membrane after wound repair in vivo.Matrix Biol. 2018 Dec;74:101-120. doi: 10.1016/j.matbio.2018.07.004. Epub 2018 Jul 5. Matrix Biol. 2018. PMID: 29981372 Free PMC article.
-
Drosophila as a model for the two myeloid blood cell systems in vertebrates.Exp Hematol. 2014 Aug;42(8):717-27. doi: 10.1016/j.exphem.2014.06.002. Epub 2014 Jun 17. Exp Hematol. 2014. PMID: 24946019 Free PMC article.
-
Polarization of the epithelial layer and apical localization of integrins are required for engulfment of apoptotic cells in the Drosophila ovary.Dis Model Mech. 2015 Dec;8(12):1603-14. doi: 10.1242/dmm.021998. Epub 2015 Sep 22. Dis Model Mech. 2015. PMID: 26398951 Free PMC article.
References
-
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

