Dissecting the influence of oxazolidinones and cyclic carbonates in sialic acid chemistry
- PMID: 22976809
- PMCID: PMC3489474
- DOI: 10.1002/anie.201204400
Dissecting the influence of oxazolidinones and cyclic carbonates in sialic acid chemistry
Abstract
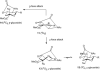
At a moment's notice: Thermal equilibration of 1 and mass spectral analysis of sialyl phosphates suggest that the 4O,5N-oxazolidinone and the 4,5-O-carbonate systems influence the anomeric effect and the mechanisms of sialidation by virtue of their dipole moment in the mean plane of the pyranose ring. The electron-withdrawing effect destabilizes 2 and promotes associative glycosylation mechanisms. TEMPO = 2,2,6,6-tetramethylpiperidine N-oxide.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
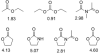

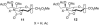



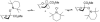
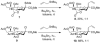

References
-
- Fraser-Reid B, Lopez C. Top. Curr. Chem. 2011;301:1–30. - PubMed
- Gomez AM. Top. Curr. Chem. 2011;301:31–68. - PubMed
- Shiao TC, Roy R. Top. Curr. Chem. 2011;301:69–108. - PubMed
- Kim KS, Suk D-H. Top. Curr. Chem. 2011;301:109–140. - PubMed
- Aubry S, Sasaki K, Sharma I, Crich D. Top. Curr. Chem. 2011;301:141–188. - PubMed
- Premathilake HD, Demchenko AV. Top. Curr. Chem. 2011;301:189–222. - PMC - PubMed
- Wu C-Y, Wong C-H. Top. Curr. Chem. 2011;301:223–252. - PubMed
- Codée JDC, Christina AE, Walvoort MTC, Overkleeft HS, van der Marel GA. Top. Curr. Chem. 2011;301:253–290. - PubMed
-
- Codée JDC, Ali A, Overkleeft HS, van der Marel GA. CR Chimie. 2011;14:178–193.
- Litjens REJN, van den Bos LJ, Codée JDC, Overkleeft HS, van der Marel G. Carbohydr. Res. 2007;342:419–429. - PubMed
-
- Crich D, Vinod AU. J. Org. Chem. 2005;70:1291–1296. - PubMed
- Manabe S, Ishii K, Ito Y. J. Am. Chem. Soc. 2006;128:10666–10667. - PubMed
- Kerns RJ, Zha C, Benakli K, Liang Y-Z. Tetrahedron Lett. 2003;44:8069–8072.
- Olsson JDM, Eriksson L, Lahmann M, Oscarson S. J. Org. Chem. 2008;73:7181–7188. - PubMed
- Satoh H, Manabe S, Ito Y, Luthi HP, Laino T, Hutter J. J. Am. Chem. Soc. 2011;133:5610–5619. - PubMed
-
- Tanaka H, Nishiura Y, Takahashi T. J. Am. Chem. Soc. 2006;128:7124–7125. - PubMed
- Farris MD, De Meo C. Tetrahedron Lett. 2007;48:1225–1227.
- Crich D, Li W. J. Org. Chem. 2007;72:2387–2391. - PMC - PubMed
- Crich D, Li W. J. Org. Chem. 2007;72:7794–7797. - PMC - PubMed
- Crich D, Wu B. Org. Lett. 2008;10:4033–4035. - PMC - PubMed
- Hsu C-H, Chu K-C, Lin Y-S, Han J-L, Peng Y-S, Ren C-T, Wu C-Y, Wong C-H. Chem. Eur. J. 2010;16:1754–1760. - PubMed
- Chu K-C, Ren C-T, Lu C-P, Hsu C-H, Sun T-H, Han J-L, Pal B, Chao T-A, Lin Y-F, Wu S-H, Wong C-H, Wu C-Y. Angew. Chem. Int. Ed. 2011;50:9391–9395. - PubMed
- Harris BN, Patel PP, Gobble CP, Stark MJ, De Meo C. Eur. J. Org. Chem. 2011:4023–4027.
- Wang Y-J, Jia J, Gu Z-Y, Liang F-F, Li R-C, Huang M-H, Xu C-S, Zhang J-X, Men Y, Xing G-W. Carbohydr. Res. 2011;346:1271–1276. - PubMed
- Crich D, Navuluri C. Angew. Chem. Int. Ed. 2010;49:3049–3052. - PMC - PubMed
- Gu Z.-y., Zhang J.-x., Guo-wen Xing G.-x. Chem. Asian J. 2012;7:1524–1528. - PubMed
-
- Noel A, Delpech B, Crich D. Org. Lett. 2012;14:1342–1345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

