Kinetic and thermodynamic effects of mutations of human sulfite oxidase
- PMID: 22976958
- PMCID: PMC3517162
- DOI: 10.1002/cbdv.201200010
Kinetic and thermodynamic effects of mutations of human sulfite oxidase
Figures
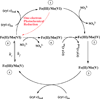
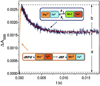
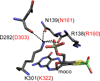


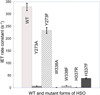

References
-
- Rupar CA, Gillett J, Gordon BA, Ramsay DA, Johnson JL, Garrett RM, Rajagopalan KV, Jung JH, Bacheyie GS, Seller AR. Neuropediatrics. 1996;27:299–304. - PubMed
-
- Schwahn BC, Galloway PG, Bowhay S, Veldman A, Santamaria JA, Schwarz G, Belaidi AA. J. Inher. Metab. Dis. 2010;33:S29–S39.
-
- Kisker C, Schindelin H, Pacheco A, Wehbi W, Garrett RM, Rajagopalan KV, Enemark JH, Rees DC. Cell. 1997;91:973–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

