FoxM1 inhibition sensitizes resistant glioblastoma cells to temozolomide by downregulating the expression of DNA-repair gene Rad51
- PMID: 22977194
- PMCID: PMC3639123
- DOI: 10.1158/1078-0432.CCR-12-0039
FoxM1 inhibition sensitizes resistant glioblastoma cells to temozolomide by downregulating the expression of DNA-repair gene Rad51
Erratum in
-
Editor's Note: FoxM1 Inhibition Sensitizes Resistant Glioblastoma Cells to Temozolomide by Downregulating the Expression of DNA-Repair Gene Rad51.Clin Cancer Res. 2025 Jul 15;31(14):3098. doi: 10.1158/1078-0432.CCR-25-1785. Clin Cancer Res. 2025. PMID: 40660829 No abstract available.
Abstract
Purpose: Recurrent glioblastoma multiforme (GBM) is characterized by resistance to radiotherapy and chemotherapy and a poor clinical prognosis. In this study, we investigated the role of the oncogenic transcription factor FoxM1 in GBM cells' resistance to alkylator temozolomide (TMZ) and its potential molecular mechanism.
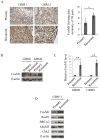
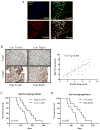
Experimental design: FoxM1 expression levels were measured by immunohistochemical analysis in 38 pairs of primary and recurrent GBM tumor samples. Expression levels were also measured in primary recurrent GBM cell lines, and their responses to TMZ were characterized. In a mechanistic study, an siRNA array was used to identify downstream genes, and a chromatin immunoprecipitation assay was used to confirm transcriptional regulation.
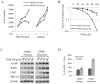
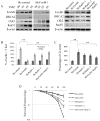
Results: Recurrent tumors that were TMZ resistant expressed higher levels of FoxM1 than did primary tumors. Recurrent GBM cell lines expressed higher levels of FoxM1 and the DNA damage repair gene Rad51 and were resistant to TMZ. TMZ treatment led to increased FoxM1 and Rad51 expression. FoxM1 knockdown inhibited Rad51 expression and sensitized recurrent GBM cells to TMZ cytotoxicity. FoxM1 directly regulated Rad51 expression through 2 FoxM1-specific binding sites in its promoter. Rad51 reexpression partially rescued TMZ resistance in FoxM1-knockdown recurrent GBM cells. A direct correlation between FoxM1 expression and Rad51 expression was evident in recurrent GBM tumor samples.
Conclusion: Targeting the FoxM1-Rad51 axis may be an effective method to reverse TMZ resistance in recurrent GBM.
©2012 AACR.
Conflict of interest statement
Conflicts of interest: There are no conflicts of interest for all authors.
Figures


References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. - PubMed
-
- Tisdale MJ. Antitumor imidazotetrazines–XV. Role of guanine O6 alkylation in the mechanism of cytotoxicity of imidazotetrazinones. Biochem Pharmacol. 1987;36:457–62. - PubMed
-
- Sobol RW. Temozolomide. In: Schwab M, editor. Encyclopedia of cancer. 2. Berlin: Springer; 2009.
-
- Wang JY, Edelmann W. Mismatch repair proteins as sensors of alkylation DNA damage. Cancer Cell. 2006;9:417–8. - PubMed
-
- Friedman HS, Johnson SP, Dong Q, Schold SC, Rasheed BK, Bigner SH, et al. Methylator resistance mediated by mismatch repair deficiency in a glioblastoma multiforme xenograft. Cancer Res. 1997;57:2933–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

