Conformational transitions underlying pore opening and desensitization in membrane-embedded Gloeobacter violaceus ligand-gated ion channel (GLIC)
- PMID: 22977232
- PMCID: PMC3481289
- DOI: 10.1074/jbc.M112.401067
Conformational transitions underlying pore opening and desensitization in membrane-embedded Gloeobacter violaceus ligand-gated ion channel (GLIC)
Abstract
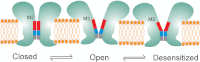
Direct structural insight into the mechanisms underlying activation and desensitization remain unavailable for the pentameric ligand-gated channel family. Here, we report the structural rearrangements underlying gating transitions in membrane-embedded GLIC, a prokaryotic homologue, using site-directed spin labeling and electron paramagnetic resonance (EPR) spectroscopy. We particularly probed the conformation of pore-lining second transmembrane segment (M2) under conditions that favor the closed and the ligand-bound desensitized states. The spin label mobility, intersubunit spin-spin proximity, and the solvent-accessibility parameters in the two states clearly delineate the underlying protein motions within M2. Our results show that during activation the extracellular hydrophobic region undergoes major changes involving an outward translational movement, away from the pore axis, leading to an increase in the pore diameter, whereas the lower end of M2 remains relatively immobile. Most notably, during desensitization, the intervening polar residues in the middle of M2 move closer to form a solvent-occluded barrier and thereby reveal the location of a distinct desensitization gate. In comparison with the crystal structure of GLIC, the structural dynamics of the channel in a membrane environment suggest a more loosely packed conformation with water-accessible intrasubunit vestibules penetrating from the extracellular end all the way to the middle of M2 in the closed state. These regions have been implicated to play a major role in alcohol and drug modulation. Overall, these findings represent a key step toward understanding the fundamentals of gating mechanisms in this class of channels.
Figures





References
-
- Hilf R. J., Dutzler R. (2009) Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457, 115–118 - PubMed
-
- Hilf R. J., Dutzler R. (2008) X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452, 375–379 - PubMed
-
- Bocquet N., Nury H., Baaden M., Le Poupon C., Changeux J. P., Delarue M., Corringer P. J. (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114 - PubMed
-
- Bocquet N., Prado de Carvalho L., Cartaud J., Neyton J., Le Poupon C., Taly A., Grutter T., Changeux J. P., Corringer P. J. (2007) A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature 445, 116–119 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

