Membrane tethering and nucleotide-dependent conformational changes drive mitochondrial genome maintenance (Mgm1) protein-mediated membrane fusion
- PMID: 22977249
- PMCID: PMC3481265
- DOI: 10.1074/jbc.C112.406769
Membrane tethering and nucleotide-dependent conformational changes drive mitochondrial genome maintenance (Mgm1) protein-mediated membrane fusion
Abstract
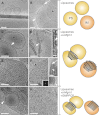
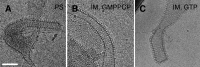
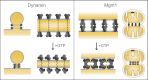
Cellular membrane remodeling events such as mitochondrial dynamics, vesicle budding, and cell division rely on the large GTPases of the dynamin superfamily. Dynamins have long been characterized as fission molecules; however, how they mediate membrane fusion is largely unknown. Here we have characterized by cryo-electron microscopy and in vitro liposome fusion assays how the mitochondrial dynamin Mgm1 may mediate membrane fusion. Using cryo-EM, we first demonstrate that the Mgm1 complex is able to tether opposing membranes to a gap of ∼15 nm, the size of mitochondrial cristae folds. We further show that the Mgm1 oligomer undergoes a dramatic GTP-dependent conformational change suggesting that s-Mgm1 interactions could overcome repelling forces at fusion sites and that ultrastructural changes could promote the fusion of opposing membranes. Together our findings provide mechanistic details of the two known in vivo functions of Mgm1, membrane fusion and cristae maintenance, and more generally shed light onto how dynamins may function as fusion proteins.
Figures

References
-
- Danino D., Hinshaw J. E. (2001) Dynamin family of mechanoenzymes. Curr. Opin. Cell Biol. 13, 454–460 - PubMed
-
- Praefcke G. J., McMahon H. T. (2004) The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 5, 133–147 - PubMed
-
- Takei K., Haucke V., Slepnev V., Farsad K., Salazar M., Chen H., De Camilli P. (1998) Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell 94, 131–141 - PubMed
-
- Sweitzer S. M., Hinshaw J. E. (1998) Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell 93, 1021–1029 - PubMed
-
- Stowell M. H., Marks B., Wigge P., McMahon H. T. (1999) Nucleotide-dependent conformational changes in dynamin: evidence for a mechanochemical molecular spring. Nat. Cell Biol. 1, 27–32 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

