Distinct expression patterns of glycoprotein hormone subunits in the lophotrochozoan Aplysia: implications for the evolution of neuroendocrine systems in animals
- PMID: 22977258
- PMCID: PMC3473217
- DOI: 10.1210/en.2012-1677
Distinct expression patterns of glycoprotein hormone subunits in the lophotrochozoan Aplysia: implications for the evolution of neuroendocrine systems in animals
Abstract
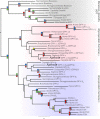
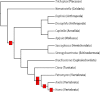
Glycoprotein hormones (GPHs) comprise a group of signaling molecules critical for major metabolic and reproductive functions. In vertebrates they include chorionic gonadotropin, LH, FSH, and TSH. The active hormones are characterized by heterodimerization between a common α and hormone-specific β subunit, which activate leucine-rich repeat-containing G protein coupled receptors. To date, genes referred to as GPHα2 and GPHβ5 have been the only glycoprotein hormone subunits identified in invertebrates, suggesting that other GPHα and GPHβ subunits diversified during vertebrate evolution. Still the functions of GPHα2 and GPHβ5 remain largely unknown for both vertebrates and invertebrates. To further understand the evolution and putative function of these subunits, we cloned and analyzed phylogenetically two glycoprotein subunits, AcaGPHα and AcaGPHβ, from the sea hare Aplysia californica. Model based three-dimensional predictions of AcaGPHβ confirm the presence of a complete cysteine knot, two hairpin loops, and a long loop. As in the human GPHβ5 subunit the seatbelt structure is absent in AcaGPHβ. We also found that AcaGPHα and AcaGPHβ subunits are expressed in larval stages of Aplysia, and we present a detailed expression map of the subunits in the adult central nervous system using in situ hybridizations. Both subunits are expressed in subpopulations of pleural and buccal mechanosensory neurons, suggesting a neuronal modulatory function of these subunits in Aplysia. Furthermore it supports the model of a relatively diffuse neuroendocrine-like system in molluscs, where specific primary sensory neurons release peptides extrasynaptically (paracrine secretion). This is in contrast to vertebrates and insects, in which releasing and stimulating factor from centralized sensory regions of the central nervous system ultimately regulate hormone release in peripheral glands.
Figures




References
-
- Eales JG. 1997. Iodine metabolism and thyroid-related functions in organisms lacking thyroid follicles: are thyroid hormones also vitamins? Proc Soc Exp Biol Med 214:302–317 - PubMed
-
- Salvator G. 1969. Thyroid hormone biosynthesis in Agnatha and Protochordata. Gen Comp Endocrinol 2:535–550
-
- Gorbman A. 1995. Olfactory origins and evolution of the brain pituitary endocrine system: facts and speculation. Gen Comp Endocrinol 97:171–178 - PubMed
-
- Heyland A, Hodin J, Reitzel AM. 2005. Hormone signaling in evolution and development: a non-model system approach. Bioessays 27:64–75 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

