Weekly paclitaxel, gemcitabine, and external irradiation followed by randomized farnesyl transferase inhibitor R115777 for locally advanced pancreatic cancer
- PMID: 22977306
- PMCID: PMC3430391
- DOI: 10.2147/OTT.S33560
Weekly paclitaxel, gemcitabine, and external irradiation followed by randomized farnesyl transferase inhibitor R115777 for locally advanced pancreatic cancer
Abstract
Purpose: The Radiation Therapy Oncology Group (RTOG) multi-institutional Phase II study 98-12, evaluating paclitaxel and concurrent radiation (RT) for locally advanced pancreatic cancer, demonstrated a median survival of 11.3 months and a 1-year survival of 43%. The purpose of the randomized Phase II study by RTOG 0020 was to evaluate the addition of weekly low- dose gemcitabine with concurrent paclitaxel/RT and to evaluate the efficacy and safety of the farnesyl transferase inhibitor R115777 following chemoradiation.
Patients and methods: Patients with unresectable, nonmetastatic adenocarcinoma of the pancreas were eligible. Patients in Arm 1 received gemcitabine, 75 mg/m(2)/week, and paclitaxel, 40 mg/m(2)/week, for 6 weeks, with 50.4 Gy radiation (CXRT). Patients in Arm 2 received an identical chemoradiation regimen but then received maintenance R115777, 300 mg twice a day for 21 days every 28 days (CXRT+R115777), until disease progression or unacceptable toxicity.
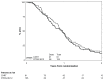
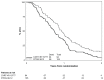
Results: One hundred ninety-five patients were entered into this study, and 184 were analyzable. Grade 4 nonhematologic toxicities occurred in less than 5% of CXRT patients. The most common grade 3/4 toxicity from R115777 was myelosuppression; however, grade 3/4 hepatic, metabolic, musculoskeletal, and neurologic toxicities were also reported. The median survival time was 11.5 months and 8.9 months for the CXRT and CXRT+R115777 arms, respectively.
Conclusions: The CXRT arm achieved a median survival of almost 1-year, supporting chemoradiation as an important therapeutic modality for locally advanced pancreatic cancer. Maintenance R115777 is not effective and is associated with a broad range of toxicities. These findings provide clinical evidence that inhibition of farnesylation affects many metabolic pathways, underscoring the challenge of developing an effective K-ras inhibitor.
Keywords: gemcitabine; irradiation; paclitaxel; pancreas cancer.
Figures

Similar articles
-
A Phase I trial of the farnesyl protein transferase inhibitor R115777 in combination with gemcitabine and cisplatin in patients with advanced cancer.Clin Cancer Res. 2003 Jul;9(7):2520-6. Clin Cancer Res. 2003. PMID: 12855626 Clinical Trial.
-
A phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest oncology group (SWOG 9924) study.Invest New Drugs. 2005 Oct;23(5):485-7. doi: 10.1007/s10637-005-2908-y. Invest New Drugs. 2005. PMID: 16133800 Clinical Trial.
-
Phase II study of external irradiation and weekly paclitaxel for nonmetastatic, unresectable pancreatic cancer: RTOG-98-12.Am J Clin Oncol. 2004 Feb;27(1):51-6. doi: 10.1097/01.coc.0000046300.88847.bf. Am J Clin Oncol. 2004. PMID: 14758134 Clinical Trial.
-
Paclitaxel as a radiation sensitizer for locally advanced pancreatic cancer.Crit Rev Oncol Hematol. 2002 Jul;43(1):57-62. doi: 10.1016/s1040-8428(01)00184-6. Crit Rev Oncol Hematol. 2002. PMID: 12098607 Review.
-
Clinical research in pancreatic cancer: the Radiation Therapy Oncology Group trials.Int J Radiat Oncol Biol Phys. 2003;56(4 Suppl):31-7. doi: 10.1016/s0360-3016(03)00446-2. Int J Radiat Oncol Biol Phys. 2003. PMID: 12826249 Review.
Cited by
-
Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer.Nat Rev Gastroenterol Hepatol. 2024 Feb;21(2):101-124. doi: 10.1038/s41575-023-00856-2. Epub 2023 Nov 30. Nat Rev Gastroenterol Hepatol. 2024. PMID: 38036745 Review.
-
Pancreatic cancer: optimizing treatment options, new, and emerging targeted therapies.Drug Des Devel Ther. 2015 Jul 7;9:3529-45. doi: 10.2147/DDDT.S60328. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26185420 Free PMC article. Review.
-
Biological determinants of radioresistance and their remediation in pancreatic cancer.Biochim Biophys Acta Rev Cancer. 2017 Aug;1868(1):69-92. doi: 10.1016/j.bbcan.2017.02.003. Epub 2017 Feb 27. Biochim Biophys Acta Rev Cancer. 2017. PMID: 28249796 Free PMC article. Review.
-
Therapeutic resistance in pancreatic ductal adenocarcinoma: Current challenges and future opportunities.World J Gastroenterol. 2021 Oct 21;27(39):6527-6550. doi: 10.3748/wjg.v27.i39.6527. World J Gastroenterol. 2021. PMID: 34754151 Free PMC article. Review.
-
Compliance with therapeutic guidelines in Radiation Therapy Oncology Group prospective gastrointestinal clinical trials.Radiother Oncol. 2012 Oct;105(1):9-13. doi: 10.1016/j.radonc.2012.09.011. Epub 2012 Oct 17. Radiother Oncol. 2012. PMID: 23084596 Free PMC article.
References
-
- Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol. 2003;21(18):3409–3414. - PubMed
-
- Huguet F, Girard N, Guerche CS, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27(13):2269–2277. - PubMed
-
- Conroy T, Desseigne M, Ychou M, et al. Randomized Phase III trial comparing FOLFIRINOX (F: 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O] versus gemcitabine [G] as first-line treatment for metastatic pancreatic adenocarcinoma (MPA): preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial. J Clin Oncol. 2010;28(Suppl, abstract 4010):S15.
-
- Bo G, De Paoli A, Innocente R, et al. Radiotherapy and continuous infusion 5-fluorouracil in patients with nonresectable pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2001;51:736–740. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

