Weekly paclitaxel, gemcitabine, and external irradiation followed by randomized farnesyl transferase inhibitor R115777 for locally advanced pancreatic cancer
- PMID: 22977306
- PMCID: PMC3430391
- DOI: 10.2147/OTT.S33560
Weekly paclitaxel, gemcitabine, and external irradiation followed by randomized farnesyl transferase inhibitor R115777 for locally advanced pancreatic cancer
Abstract
Purpose: The Radiation Therapy Oncology Group (RTOG) multi-institutional Phase II study 98-12, evaluating paclitaxel and concurrent radiation (RT) for locally advanced pancreatic cancer, demonstrated a median survival of 11.3 months and a 1-year survival of 43%. The purpose of the randomized Phase II study by RTOG 0020 was to evaluate the addition of weekly low- dose gemcitabine with concurrent paclitaxel/RT and to evaluate the efficacy and safety of the farnesyl transferase inhibitor R115777 following chemoradiation.
Patients and methods: Patients with unresectable, nonmetastatic adenocarcinoma of the pancreas were eligible. Patients in Arm 1 received gemcitabine, 75 mg/m(2)/week, and paclitaxel, 40 mg/m(2)/week, for 6 weeks, with 50.4 Gy radiation (CXRT). Patients in Arm 2 received an identical chemoradiation regimen but then received maintenance R115777, 300 mg twice a day for 21 days every 28 days (CXRT+R115777), until disease progression or unacceptable toxicity.
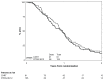
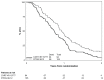
Results: One hundred ninety-five patients were entered into this study, and 184 were analyzable. Grade 4 nonhematologic toxicities occurred in less than 5% of CXRT patients. The most common grade 3/4 toxicity from R115777 was myelosuppression; however, grade 3/4 hepatic, metabolic, musculoskeletal, and neurologic toxicities were also reported. The median survival time was 11.5 months and 8.9 months for the CXRT and CXRT+R115777 arms, respectively.
Conclusions: The CXRT arm achieved a median survival of almost 1-year, supporting chemoradiation as an important therapeutic modality for locally advanced pancreatic cancer. Maintenance R115777 is not effective and is associated with a broad range of toxicities. These findings provide clinical evidence that inhibition of farnesylation affects many metabolic pathways, underscoring the challenge of developing an effective K-ras inhibitor.
Keywords: gemcitabine; irradiation; paclitaxel; pancreas cancer.
Figures

References
-
- Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol. 2003;21(18):3409–3414. - PubMed
-
- Huguet F, Girard N, Guerche CS, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27(13):2269–2277. - PubMed
-
- Conroy T, Desseigne M, Ychou M, et al. Randomized Phase III trial comparing FOLFIRINOX (F: 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O] versus gemcitabine [G] as first-line treatment for metastatic pancreatic adenocarcinoma (MPA): preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial. J Clin Oncol. 2010;28(Suppl, abstract 4010):S15.
-
- Bo G, De Paoli A, Innocente R, et al. Radiotherapy and continuous infusion 5-fluorouracil in patients with nonresectable pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2001;51:736–740. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

