Thalidomide and irradiation combination therapy increases substance P levels in vitro
- PMID: 22977536
- PMCID: PMC3440713
- DOI: 10.3892/etm.2011.216
Thalidomide and irradiation combination therapy increases substance P levels in vitro
Abstract
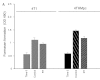
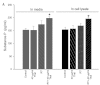
Thalidomide is an anti-angiogenic agent that is used in the treatment of cancer. However, in many cases, particularly in patients with breast cancer, thalidomide treatment alone is insufficient and must be combined with other drugs or therapies. In the clinical setting, thalidomide is most commonly used in combination with radiation therapy. However, the exact mechanisms of its effect are unkown. Radiotherapy alters the expression of substance P, which is considered a crucial pro-angiogenic peptide. To determine whether thalidomide and radiotherapy in combination overcome the limitations of each as monotherapy, we examined the effects of the combination on the growth of breast cancer cells as well as on the expression of substance P in vitro. Mouse breast cancer cells (4T1) and cells produced from metastatic lesions (4THMpc) were treated with radiotherapy (RT) (45 Gy) alone, thalidomide (Thal) (40 μg/ml) alone or combination therapy (40 μg/ml Thal + 45 Gy RT), and compared with control cells. MTS, Live/Dead and trypan blue exclusion assays were used to evaluate the cytotoxic effects of the treatments. The levels of substance P in the conditioned media and in the cell lysates were determined by a substance P ELISA kit, and changes in the protein content were analyzed by Western blotting. Thalidomide alone resulted in a significant inhibition in the growth of the 4T1 (34.1%) and 4THMpc (52.6%) cell lines. RT alone inhibited the growth of the 4T1 (19.2%) and 4THMpc (23.31%) cell lines. The combination therapy enhanced the growth inhibition noted in the 4T1 (47.9%) and 4THMpc (62.03%) cell lines. The expression of substance P in the conditioned media and in the cell lysates increased within 72 h of RT. This increase was significantly enhanced with the combination therapy. These data indicate that thalidomide inhibits breast cancer cell growth and potentiates the anti-tumor effects of radiation at appropriate doses.
Figures






References
-
- Polyak K. On the birth of breast cancer. Biochim Biophys Acta. 2001;1552:1–13. - PubMed
-
- Goss PE. Breast cancer prevention – clinical trials strategies involving aromatase inhibitors. J Steroid Biochem Mol Biol. 2003;86:487–493. - PubMed
-
- Dong X, Han ZC, Yang R. Angiogenesis and antiangiogenic therapy in hematologic malignancies. Crit Rev Oncol Hematol. 2007;62:105–118. - PubMed
-
- Schneider BP, Miller KD. Angiogenesis of breast cancer. J Clin Oncol. 2005;23:1782–1790. - PubMed
LinkOut - more resources
Full Text Sources
