Cell budding from pre-invasive tumors: Intrinsic precursor of invasive breast lesions?
- PMID: 22977553
- PMCID: PMC3440761
- DOI: 10.3892/etm.2011.251
Cell budding from pre-invasive tumors: Intrinsic precursor of invasive breast lesions?
Abstract
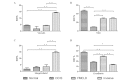
Our previous studies showed that in patients with ductal carcinoma in situ (DCIS) of the breast, the tumor cells that overlie focal myoepithelial cell layer disruptions (FMCLDs) are generally arranged as finger-like projections that bud into the stroma. These budding cells have significantly more genetic instability and invasion-related gene expression, and less estrogen receptor (ER) expression, than their epithelial cell counterparts. This study aimed to assess these cells for potential molecular markers that are uniquely associated with cell adhesion and motility. Seventeen ER-positive DCIS cases were screened by immunostaining for ER, and 7 cases which harbored FMCLD lesions were used to examine the expression of the potential markers. Two cases with both DCIS and invasive lesions were selected for comparing the differences in molecular expression between these lesion types. The results showed that expression levels of talin, E-cadherin and focal adhesion kinase (FAK) in tumor cells overlying FMCLDs were higher than those within the corresponding duct. Integrin β1 staining was detected only in a small number of the tumor cells overlying the FMCLDs. Vinculin staining was weak (18%) or not detected (82%), and no expression was found in the tumor cells within the corresponding duct or in the pure isolated DCIS. By contrast, the expression levels of talin, vinculin and integrin β1 in the invasive tumors were distinctly higher than those in DCIS, and the expression of FAK and E-cadherin was lower. Using electron microscopy, we found that the tight junctions between tumor cells overlying the FMCLDs were reduced compared to the adjacent tumor cells in the lumen. These results indicate that the tumor cells overlying FMCLDs are likely to represent the specific precursors of invasive breast lesions. Our findings may also facilitate the identification of specific targets for further molecular profiling, which will more completely characterize this important cell population.
Figures


References
-
- Beckmann MW, Niederacher D, Schnurch HG, Gusterson BA, Bender HG. Multistep carcinogenesis of breast cancer and tumour heterogeneity. J Mol Med. 1997;75:429–439. - PubMed
-
- Schmitt FC. Multistep progression from an oestrogen-dependent growth towards an autonomous growth in breast carcinogenesis. Eur J Cancer. 1995;31A:2049–2052. - PubMed
-
- Tsubura A, Shikata N, Inui T, et al. Immunohistochemical localization of myoepithelial cells and basement membrane in normal, benign and malignant human breast lesions. Virchows Arch A Pathol Anat Histopathol. 1988;413:133–139. - PubMed
-
- Jolicoeur F, Seemayer TA, Gabbiani G, et al. Multifocal, nascent, and invasive myoepithelial carcinoma (malignant myoepithelioma) of the breast: an immunohistochemical and ultrastructural study. Int J Surg Pathol. 2002;10:281–291. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
