Interleukin-28B polymorphisms are associated with an early viral response in patients receiving hepatitis C therapy
- PMID: 22977564
- PMCID: PMC3440742
- DOI: 10.3892/etm.2011.250
Interleukin-28B polymorphisms are associated with an early viral response in patients receiving hepatitis C therapy
Abstract
Prediction of the efficacy of pegylated interferon (PEG-IFN) plus ribavirin (RBV) therapy against hepatitis C (HCV) infection is valuable for determining its applications. This study investigated the relationship between the early response of HCV to PEG-IFN/RBV therapy and the inter-leukin (IL)-28B genetic polymorphism in patients with HCV infection. The genotypes of IL-28B rs8099917 T>G single nucleotide polymorphism were determined in 144 patients with HCV infection. Among them, 59 were treated with PEG-IFN/RBV. The frequency of IL-28B TT homozygosity was 75.2% in patients with HCV serotype 1 and 84.6% in patients with serotype 2. Multivariate analysis showed that IL-28B TT homozygosity (P=0.014) and the platelets number (P=0.030) was associated with the early suppression of HCV-RNA at 12 weeks after the start of PEG-IFN/RBV therapy. The IL-28B polymorphism was a significant pre-treatment predictor of the response to PEG-IFN/RBV therapy in patients with HCV infection.
Figures
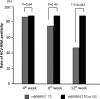
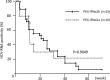
References
-
- Alavian SM, Tabatabaei SV, Keshvari M, et al. Peginterferon alpha-2a and ribavirin treatment of patients with haemophilia and hepatitis C virus infection: a single-centre study of 367 cases. Liver Int. 2010;30:1173–1180. - PubMed
-
- Welzel TM, Morgan TR, Bonkovsky HL, et al. Variants in interferon-alpha pathway genes and response to pegylated interferon-alpha2a plus ribavirin for treatment of chronic hepatitis C virus infection in the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology. 2009;49:1847–1858. - PMC - PubMed
-
- Kurosaki M, Tanaka Y, Nishida N, et al. Pre-treatment prediction of response to pegylated-interferon plus ribavirin for chronic hepatitis C using genetic polymorphism in IL28B and viral factors. J Hepatol. 2011;54:439–448. - PubMed
-
- Yasui K, Harano Y, Mitsuyoshi H, et al. Steatosis and hepatic expression of genes regulating lipid metabolism in Japanese patients infected with hepatitis C virus. J Gastroenterol. 2010;45:95–104. - PubMed
-
- Ochi H, Maekawa T, Abe H, et al. IL-28B predicts response to chronic hepatitis C therapy-fine-mapping and replication study in Asian populations. J Gen Virol. Jan. 2011. (E-pub ahead of print). - PubMed
LinkOut - more resources
Full Text Sources
