Structured illumination of the interface between centriole and peri-centriolar material
- PMID: 22977736
- PMCID: PMC3438536
- DOI: 10.1098/rsob.120104
Structured illumination of the interface between centriole and peri-centriolar material
Abstract
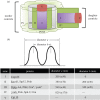
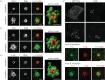
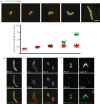
The increase in centrosome size in mitosis was described over a century ago, and yet it is poorly understood how centrioles, which lie at the core of centrosomes, organize the pericentriolar material (PCM) in this process. Now, structured illumination microscopy reveals in Drosophila that, before clouds of PCM appear, its proteins are closely associated with interphase centrioles in two tube-like layers: an inner layer occupied by centriolar microtubules, Sas-4, Spd-2 and Polo kinase; and an outer layer comprising Pericentrin-like protein (Dplp), Asterless (Asl) and Plk4 kinase. Centrosomin (Cnn) and γ-tubulin associate with this outer tube in G2 cells and, upon mitotic entry, Polo activity is required to recruit them together with Spd-2 into PCM clouds. Cnn is required for Spd-2 to expand into the PCM during this maturation process but can itself contribute to PCM independently of Spd-2. By contrast, the centrioles of spermatocytes elongate from a pre-existing proximal unit during the G2 preceding meiosis. Sas-4 is restricted to the microtubule-associated, inner cylinder and Dplp and Cnn to the outer cylinder of this proximal part. γ-Tubulin and Asl associate with the outer cylinder and Spd-2 with the inner cylinder throughout the entire G2 centriole. Although they occupy different spatial compartments on the G2 centriole, Cnn, Spd-2 and γ-tubulin become diminished at the centriole upon entry into meiosis to become part of PCM clouds.
Keywords: Drosophila; centriole; pericentriolar material; super resolution microscopy.
Figures



References
-
- Wilson EB. 1896; 1900; 1925. The cell in development and inheritance. New York, NY: Macmillan
-
- Boveri T. 1914. Zur Frage der Entstehung maligner Tumoren. Jena, Germany: Gustav Fischer Verlag
-
- Boveri T. 1888. Zellenstudien II. Die Befruchtung und Teilung des Eies von Ascaris megalocephala. Jena Zeit. Naturw. 22, 685–882
-
- Strnad P, Gonczy P. 2008. Mechanisms of procentriole formation. Trends Cell Biol. 18, 389–39610.1016/j.tcb.2008.06.004 (doi:10.1016/j.tcb.2008.06.004) - DOI - DOI - PubMed
-
- Mahen R, Venkitaraman AR. 2012. Pattern formation in centrosome assembly. Curr. Opin. Cell Biol. 24, 14–2310.1016/j.ceb.2011.12.012 (doi:10.1016/j.ceb.2011.12.012) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
