Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation
- PMID: 22978461
- PMCID: PMC3445044
- DOI: 10.1111/xen.12000
Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation
Abstract
Background: Allogeneic mesenchymal stem (stromal) cells (MSC) are a promising therapy for various pathological conditions. Genetically modified pig MSC have been demonstrated to downregulate the human T-cell response to pig antigens in vitro. Before genetically modified pig MSC can be used clinically, however, evidence needs to be provided to indicate whether they will survive in a human (xenogeneic) host.
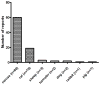
Literature search and results: A literature search through the end of 2011 identified 94 reports of the in vivo cross-species administration of MSC in a variety of experimental models. The majority (n = 89) involved the use of human MSC in various other species, with an occasional study using pig, rat, or guinea-pig MSC. When human MSC were used, they were largely derived from the bone marrow, adipose tissue, or umbilical cord blood. The routes of administration were varied, although almost half of the studies utilized the intravenous route. In 88 experiments (93.6%), there was evidence that the MSC engrafted and functioned across the species barrier, and in only six cases (6.4%) was there evidence of failure to function. Importantly, MSC function was confirmed in several different cross-species models. For example, human MSC functioned in no fewer than seven different recipient species.
Conclusions: The data provided by this literature search strengthen the hypothesis that pig MSC will function satisfactorily in a different species, for example, humans. The data also suggest that our own in vitro observations on the efficacy of pig MSC in downregulating the strength of the human T-cell response to pig antigens will likely be reproduced in vivo in pre-clinical large animal models and in clinical trials.
© 2012 John Wiley & Sons A/S.
Conflict of interest statement
The authors have no financial or other conflict of interest.
Figures
Similar articles
-
The potential role of genetically-modified pig mesenchymal stromal cells in xenotransplantation.Stem Cell Rev Rep. 2014 Feb;10(1):79-85. doi: 10.1007/s12015-013-9478-8. Stem Cell Rev Rep. 2014. PMID: 24142483 Free PMC article. Review.
-
Adipose-derived mesenchymal stromal cells from genetically modified pigs: immunogenicity and immune modulatory properties.Cytotherapy. 2012 Apr;14(4):494-504. doi: 10.3109/14653249.2011.651529. Epub 2012 Jan 23. Cytotherapy. 2012. PMID: 22264190 Free PMC article.
-
Intra-bone marrow cotransplantation of donor mesenchymal stem cells in pig-to-NOD/SCID mouse bone marrow transplantation facilitates short-term xenogeneic hematopoietic engraftment.Transplant Proc. 2008 Mar;40(2):574-7. doi: 10.1016/j.transproceed.2008.02.012. Transplant Proc. 2008. PMID: 18374132
-
Efficacy of Neonatal Porcine Bone Marrow-Derived Mesenchymal Stem Cell Xenotransplantation for the Therapy of Hind Limb Lymphedema in Mice.Cell Transplant. 2024 Jan-Dec;33:9636897241260195. doi: 10.1177/09636897241260195. Cell Transplant. 2024. PMID: 38867486 Free PMC article.
-
Experimental hepatocyte xenotransplantation--a comprehensive review of the literature.Xenotransplantation. 2015 Jul-Aug;22(4):239-48. doi: 10.1111/xen.12170. Epub 2015 May 7. Xenotransplantation. 2015. PMID: 25950141 Free PMC article. Review.
Cited by
-
Xenogenic Application of Human Placenta-Derived Mesenchymal Stromal Cells in a Porcine Large Animal Model.Cell Transplant. 2024 Jan-Dec;33:9636897241226737. doi: 10.1177/09636897241226737. Cell Transplant. 2024. PMID: 38323325 Free PMC article.
-
Infusing Mesenchymal Stromal Cells into Porcine Kidneys during Normothermic Machine Perfusion: Intact MSCs Can Be Traced and Localised to Glomeruli.Int J Mol Sci. 2019 Jul 23;20(14):3607. doi: 10.3390/ijms20143607. Int J Mol Sci. 2019. PMID: 31340593 Free PMC article.
-
Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: a preliminary study on biglycan-deficient murine model of chronic disc degeneration.Arthritis Res Ther. 2014 Oct 8;16(5):457. doi: 10.1186/s13075-014-0457-5. Arthritis Res Ther. 2014. PMID: 25293819 Free PMC article.
-
Utility of a Mouse Model of Osteoarthritis to Demonstrate Cartilage Protection by IFNγ-Primed Equine Mesenchymal Stem Cells.Front Immunol. 2016 Sep 27;7:392. doi: 10.3389/fimmu.2016.00392. eCollection 2016. Front Immunol. 2016. PMID: 27729913 Free PMC article.
-
Improvement of cardiac function by placenta-derived mesenchymal stem cells does not require permanent engraftment and is independent of the insulin signaling pathway.Stem Cell Res Ther. 2014 Aug 21;5(4):102. doi: 10.1186/scrt490. Stem Cell Res Ther. 2014. PMID: 25145631 Free PMC article.
References
-
- SORDI V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87:S42–45. - PubMed
-
- LE BLANC K, RASMUSSON I, SUNDBERG B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. - PubMed
-
- LE BLANC K, FRASSONI F, BALL L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. - PubMed
-
- MACDONALD GI, AUGELLO A, DEBARIC Role of mesenchymal stem cells in reestablishing immunologic tolerance in autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:2547–2557. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

