Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation
- PMID: 22978461
- PMCID: PMC3445044
- DOI: 10.1111/xen.12000
Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation
Abstract
Background: Allogeneic mesenchymal stem (stromal) cells (MSC) are a promising therapy for various pathological conditions. Genetically modified pig MSC have been demonstrated to downregulate the human T-cell response to pig antigens in vitro. Before genetically modified pig MSC can be used clinically, however, evidence needs to be provided to indicate whether they will survive in a human (xenogeneic) host.
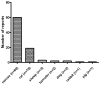
Literature search and results: A literature search through the end of 2011 identified 94 reports of the in vivo cross-species administration of MSC in a variety of experimental models. The majority (n = 89) involved the use of human MSC in various other species, with an occasional study using pig, rat, or guinea-pig MSC. When human MSC were used, they were largely derived from the bone marrow, adipose tissue, or umbilical cord blood. The routes of administration were varied, although almost half of the studies utilized the intravenous route. In 88 experiments (93.6%), there was evidence that the MSC engrafted and functioned across the species barrier, and in only six cases (6.4%) was there evidence of failure to function. Importantly, MSC function was confirmed in several different cross-species models. For example, human MSC functioned in no fewer than seven different recipient species.
Conclusions: The data provided by this literature search strengthen the hypothesis that pig MSC will function satisfactorily in a different species, for example, humans. The data also suggest that our own in vitro observations on the efficacy of pig MSC in downregulating the strength of the human T-cell response to pig antigens will likely be reproduced in vivo in pre-clinical large animal models and in clinical trials.
© 2012 John Wiley & Sons A/S.
Conflict of interest statement
The authors have no financial or other conflict of interest.
Figures
References
-
- SORDI V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87:S42–45. - PubMed
-
- LE BLANC K, RASMUSSON I, SUNDBERG B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. - PubMed
-
- LE BLANC K, FRASSONI F, BALL L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. - PubMed
-
- MACDONALD GI, AUGELLO A, DEBARIC Role of mesenchymal stem cells in reestablishing immunologic tolerance in autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:2547–2557. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

