Genome wide identification of chilling responsive microRNAs in Prunus persica
- PMID: 22978558
- PMCID: PMC3463484
- DOI: 10.1186/1471-2164-13-481
Genome wide identification of chilling responsive microRNAs in Prunus persica
Abstract
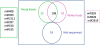
Background: MicroRNAs (miRNAs) are small RNAs (sRNAs) approximately 21 nucleotides in length that negatively control gene expression by cleaving or inhibiting the translation of target gene transcripts. Within this context, miRNAs and siRNAs are coming to the forefront as molecular mediators of gene regulation in plant responses to annual temperature cycling and cold stress. For this reason, we chose to identify and characterize the conserved and non-conserved miRNA component of peach (Prunus persica (L.) Batsch) focusing our efforts on both the recently released whole genome sequence of peach and sRNA transcriptome sequences from two tissues representing non-dormant leaves and dormant leaf buds. Conserved and non-conserved miRNAs, and their targets were identified. These sRNA resources were used to identify cold-responsive miRNAs whose gene targets co-localize with previously described QTLs for chilling requirement (CR).
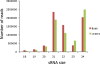
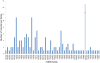
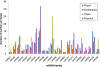
Results: Analysis of 21 million peach sRNA reads allowed us to identify 157 and 230 conserved and non-conserved miRNA sequences. Among the non-conserved miRNAs, we identified 205 that seem to be specific to peach. Comparative genome analysis between peach and Arabidopsis showed that conserved miRNA families, with the exception of miR5021, are similar in size. Sixteen of these conserved miRNA families are deeply rooted in land plant phylogeny as they are present in mosses and/or lycophytes. Within the other conserved miRNA families, five families (miR1446, miR473, miR479, miR3629, and miR3627) were reported only in tree species (Populustrichocarpa, Citrus trifolia, and Prunus persica). Expression analysis identified several up-regulated or down-regulated miRNAs in winter buds versus young leaves. A search of the peach proteome allowed the prediction of target genes for most of the conserved miRNAs and a large fraction of non-conserved miRNAs. A fraction of predicted targets in peach have not been previously reported in other species. Several conserved and non-conserved miRNAs and miRNA-regulated genes co-localize with Quantitative Trait Loci (QTLs) for chilling requirement (CR-QTL) and bloom date (BD-QTL).
Conclusions: In this work, we identified a large set of conserved and non-conserved miRNAs and describe their evolutionary footprint in angiosperm lineages. Several of these miRNAs were induced in winter buds and co-localized with QTLs for chilling requirement and bloom date thus making their gene targets potential candidates for mediating plant responses to cold stress. Several peach homologs of genes participating in the regulation of vernalization in Arabidopsis were identified as differentially expressed miRNAs targets, potentially linking these gene activities to cold responses in peach dormant buds. The non-conserved miRNAs may regulate cellular, physiological or developmental processes specific to peach and/or other tree species.
Figures

References
-
- Mallory AC, Vaucheret H. Functions of microRNAs and related small RNAs in plants. Nat Genet. 2006;38(Suppl):S31–S36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

