Probing the mycobacterial trehalome with bioorthogonal chemistry
- PMID: 22978752
- PMCID: PMC3466019
- DOI: 10.1021/ja3062419
Probing the mycobacterial trehalome with bioorthogonal chemistry
Abstract
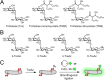
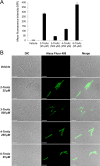
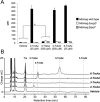
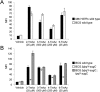
Mycobacteria, including the pathogen Mycobacterium tuberculosis, use the non-mammalian disaccharide trehalose as a precursor for essential cell-wall glycolipids and other metabolites. Here we describe a strategy for exploiting trehalose metabolic pathways to label glycolipids in mycobacteria with azide-modified trehalose (TreAz) analogues. Subsequent bioorthogonal ligation with alkyne-functionalized probes enabled detection and visualization of cell-surface glycolipids. Characterization of the metabolic fates of four TreAz analogues revealed unique labeling routes that can be harnessed for pathway-targeted investigation of the mycobacterial trehalome.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

