Isolation, analysis and antimicrobial activity of the acidic fractions of Mastic, Kurdica, Mutica and Cabolica gums from genus Pistacia
- PMID: 22980113
- PMCID: PMC4777032
- DOI: 10.5539/gjhs.v4n1p217
Isolation, analysis and antimicrobial activity of the acidic fractions of Mastic, Kurdica, Mutica and Cabolica gums from genus Pistacia
Abstract
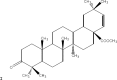
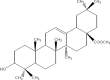
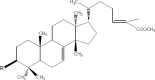
The chemical entities of Mastic, Kurdica, Mutica and Cabolica gums from genus Pistacia have been isolated and characterised by GC-Mass Spectrometry, High Performance Liquid Chromatography and Column Chromatography. These chemical entities were screened for anti-microbial activities against nine strains of Helicobacter pylori and some other Gram-negative and Gram-positive bacteria. The most bioactive components were structurally analysed. These components mimic steroid compounds, in particular, the known antibiotic Fusidic acid. Some of these chemical entities have produced promising data that could lead to the development of a novel class of antimicrobial agents that may have application in the treatment of infectious disease. Kill kinetics have been also performed, and the produced data were evaluated by Generalized Multiplicative Analysis Of Variance (GEMANOVA) for the bactericidal and bacteriostatic activities which can be clinically significant. The isolated components were all bactericidal.
Figures
References
-
- Akpuaka M, Orakwue F, Nnadozie U, et al. Preliminary phytochemical and antibacterial activity screening of Bryophyllum pinnatum extracts. Journal of Chemical Society of Nigeria. 2003;28:11–14.
-
- Al-Trabeen F, Mohammad K, Victor W, et al. New steroidal saponins from the sponge Erylus. Coden Agfuar. 2004;13:17–27.
-
- Bangwei D, Uale T, Thomas O, et al. Savage synthesis and characterization of peptide-cationic steroid antibiotic conjugates. Organic Letters. 2004;6:3433–3436. - PubMed
-
- Barton D. H. R, Seaone E. The constitution and stereochemistry of masticadienonic acid. Journal of Chemical Society. 1956:4150–4158.
-
- Caputo R, Monaco P, Mangoni L, et al. Triterpenes from the bled resin of Pistacia vera. Phytochemistry. 1978:815–817.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous