Efferocytosis is an innate antibacterial mechanism
- PMID: 22980326
- PMCID: PMC3517204
- DOI: 10.1016/j.chom.2012.06.010
Efferocytosis is an innate antibacterial mechanism
Abstract
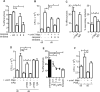
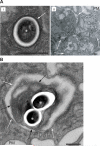
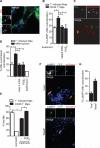
Mycobacterium tuberculosis persists within macrophages in an arrested phagosome and depends upon necrosis to elude immunity and disseminate. Although apoptosis of M. tuberculosis-infected macrophages is associated with reduced bacterial growth, the bacteria are relatively resistant to other forms of death, leaving the mechanism underlying this observation unresolved. We find that after apoptosis, M. tuberculosis-infected macrophages are rapidly taken up by uninfected macrophages through efferocytosis, a dedicated apoptotic cell engulfment process. Efferocytosis of M. tuberculosis sequestered within an apoptotic macrophage further compartmentalizes the bacterium and delivers it along with the apoptotic cell debris to the lysosomal compartment. M. tuberculosis is killed only after efferocytosis, indicating that apoptosis itself is not intrinsically bactericidal but requires subsequent phagocytic uptake and lysosomal fusion of the apoptotic body harboring the bacterium. While efferocytosis is recognized as a constitutive housekeeping function of macrophages, these data indicate that it can also function as an antimicrobial effector mechanism.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Comment in
-
"With a little help from my friends": efferocytosis as an antimicrobial mechanism.Cell Host Microbe. 2012 Sep 13;12(3):261-3. doi: 10.1016/j.chom.2012.08.008. Cell Host Microbe. 2012. PMID: 22980322 Free PMC article.
-
Host response: double trouble for TB.Nat Rev Microbiol. 2012 Nov;10(11):730. doi: 10.1038/nrmicro2908. Nat Rev Microbiol. 2012. PMID: 23070551 No abstract available.
References
-
- Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. - PubMed
Publication types
MeSH terms
Grants and funding
- DP2-0D001378/DP/NCCDPHP CDC HHS/United States
- R56 AI084161/AI/NIAID NIH HHS/United States
- R01AI072143/AI/NIAID NIH HHS/United States
- R56AI084161/AI/NIAID NIH HHS/United States
- UL1 RR029882/RR/NCRR NIH HHS/United States
- TL1 RR029881/RR/NCRR NIH HHS/United States
- T32-AI07387/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- 5P30AI060354/AI/NIAID NIH HHS/United States
- R01 AI098637/AI/NIAID NIH HHS/United States
- T32 AI007387/AI/NIAID NIH HHS/United States
- R01 AI072143/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

