Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages
- PMID: 22980327
- PMCID: PMC3638950
- DOI: 10.1016/j.chom.2012.07.009
Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages
Abstract
Neutrophils are typically the first responders in host defense against invading pathogens, which they destroy by both oxidative and nonoxidative mechanisms. However, despite a longstanding recognition of neutrophil presence at disease sites in tuberculosis, their role in defense against mycobacteria is unclear. Here we exploit the genetic tractability and optical transparency of zebrafish to monitor neutrophil behavior and its consequences during infection with Mycobacterium marinum, a natural fish pathogen. In contrast to macrophages, neutrophils do not interact with mycobacteria at initial infection sites. Neutrophils are subsequently recruited to the nascent granuloma in response to signals from dying infected macrophages within the granuloma, which they phagocytose. Some neutrophils then rapidly kill the internalized mycobacteria through NADPH oxidase-dependent mechanisms. Our results provide a mechanistic link to the observed patterns of neutrophils in human tuberculous granulomas and the susceptibility of humans with chronic granulomatous disease to mycobacterial infection.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


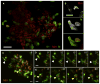
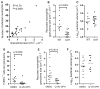


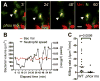
Comment in
-
"With a little help from my friends": efferocytosis as an antimicrobial mechanism.Cell Host Microbe. 2012 Sep 13;12(3):261-3. doi: 10.1016/j.chom.2012.08.008. Cell Host Microbe. 2012. PMID: 22980322 Free PMC article.
-
Host response: double trouble for TB.Nat Rev Microbiol. 2012 Nov;10(11):730. doi: 10.1038/nrmicro2908. Nat Rev Microbiol. 2012. PMID: 23070551 No abstract available.
References
-
- Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. - PubMed
-
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

