A neuron-specific role for autophagy in antiviral defense against herpes simplex virus
- PMID: 22980330
- PMCID: PMC3454454
- DOI: 10.1016/j.chom.2012.07.013
A neuron-specific role for autophagy in antiviral defense against herpes simplex virus
Abstract
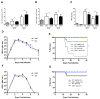
Type I interferons (IFNs) are considered to be the universal mechanism by which viral infections are controlled. However, many IFN-stimulated genes (ISGs) rely on antiviral pathways that are toxic to host cells, which may be detrimental in nonrenewable cell types, such as neurons. We show that dorsal root ganglionic (DRG) neurons produced little type I IFNs in response to infection with a neurotropic virus, herpes simplex type 1 (HSV-1). Further, type I IFN treatment failed to completely block HSV-1 replication or to induce IFN-primed cell death in neurons. We found that DRG neurons required autophagy to limit HSV-1 replication both in vivo and in vitro. In contrast, mucosal epithelial cells and other mitotic cells responded robustly to type I IFNs and did not require autophagy to control viral replication. These findings reveal a fundamental difference in the innate antiviral strategies employed by neurons and mitotic cells to control HSV-1 infection.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures
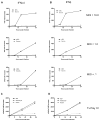
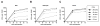
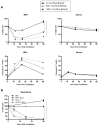
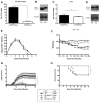

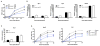
Similar articles
-
Cell type-dependent requirement of autophagy in HSV-1 antiviral defense.Autophagy. 2013 Feb 1;9(2):236-8. doi: 10.4161/auto.22506. Epub 2012 Oct 24. Autophagy. 2013. PMID: 23095715 Free PMC article.
-
Herpes Simplex Virus and Interferon Signaling Induce Novel Autophagic Clusters in Sensory Neurons.J Virol. 2016 Apr 14;90(9):4706-4719. doi: 10.1128/JVI.02908-15. Print 2016 May. J Virol. 2016. PMID: 26912623 Free PMC article.
-
The ESCRT-Related ATPase Vps4 Is Modulated by Interferon during Herpes Simplex Virus 1 Infection.mBio. 2019 Mar 5;10(2):e02567-18. doi: 10.1128/mBio.02567-18. mBio. 2019. PMID: 30837340 Free PMC article.
-
Herpes Simplex Virus Type 1 Interactions with the Interferon System.Int J Mol Sci. 2020 Jul 21;21(14):5150. doi: 10.3390/ijms21145150. Int J Mol Sci. 2020. PMID: 32708188 Free PMC article. Review.
-
Autophagy and viral neurovirulence.Cell Microbiol. 2008 Sep;10(9):1747-56. doi: 10.1111/j.1462-5822.2008.01175.x. Epub 2008 May 22. Cell Microbiol. 2008. PMID: 18503639 Free PMC article. Review.
Cited by
-
Autophagy and the effects of its inhibition on varicella-zoster virus glycoprotein biosynthesis and infectivity.J Virol. 2014 Jan;88(2):890-902. doi: 10.1128/JVI.02646-13. Epub 2013 Nov 6. J Virol. 2014. PMID: 24198400 Free PMC article.
-
A neuron-specific host microRNA targets herpes simplex virus-1 ICP0 expression and promotes latency.Cell Host Microbe. 2014 Apr 9;15(4):446-56. doi: 10.1016/j.chom.2014.03.004. Cell Host Microbe. 2014. PMID: 24721573 Free PMC article.
-
Human TBK1: A Gatekeeper of Neuroinflammation.Trends Mol Med. 2016 Jun;22(6):511-527. doi: 10.1016/j.molmed.2016.04.006. Trends Mol Med. 2016. PMID: 27211305 Free PMC article. Review.
-
Digesting the crisis: autophagy and coronaviruses.Microb Cell. 2020 May 4;7(5):119-128. doi: 10.15698/mic2020.05.715. Microb Cell. 2020. PMID: 32391393 Free PMC article.
-
Innate defense mechanisms against HSV-1 infection in the target tissues, skin and brain.J Neurovirol. 2016 Oct;22(5):641-649. doi: 10.1007/s13365-016-0440-9. Epub 2016 Apr 20. J Neurovirol. 2016. PMID: 27098517
References
-
- Barber GN. Host defense, viruses and apoptosis. Cell Death and Differentiation. 2001;8:113. - PubMed
-
- Brot MD, Rall GF, Oldstone MBA, Koob GF, Gold LH. Deficits in discriminated learning remain despite clearance of long-term persistent viral infection in mice. Journal of NeuroVirology. 1997;3:265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

