Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure
- PMID: 22980982
- PMCID: PMC3445031
- DOI: 10.1016/j.cell.2012.07.029
Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure
Abstract
Diabetes is associated with β cell failure. But it remains unclear whether the latter results from reduced β cell number or function. FoxO1 integrates β cell proliferation with adaptive β cell function. We interrogated the contribution of these two processes to β cell dysfunction, using mice lacking FoxO1 in β cells. FoxO1 ablation caused hyperglycemia with reduced β cell mass following physiologic stress, such as multiparity and aging. Surprisingly, lineage-tracing experiments demonstrated that loss of β cell mass was due to β cell dedifferentiation, not death. Dedifferentiated β cells reverted to progenitor-like cells expressing Neurogenin3, Oct4, Nanog, and L-Myc. A subset of FoxO1-deficient β cells adopted the α cell fate, resulting in hyperglucagonemia. Strikingly, we identify the same sequence of events as a feature of different models of murine diabetes. We propose that dedifferentiation trumps endocrine cell death in the natural history of β cell failure and suggest that treatment of β cell dysfunction should restore differentiation, rather than promoting β cell replication.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


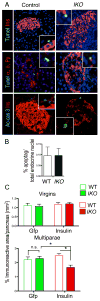
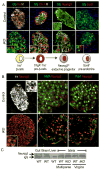



Comment in
-
Diabetic β Cells: To Be or Not To Be?Cell. 2012 Sep 14;150(6):1103-4. doi: 10.1016/j.cell.2012.08.021. Cell. 2012. PMID: 22980973 Free PMC article.
References
-
- Accili D, Ahren B, Boitard C, Cerasi E, Henquin JC, Seino S. What ails the beta-cell? Diabetes Obes Metab. 2010;12(Suppl 2):1–3. - PubMed
-
- Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, Jose PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. - PubMed
-
- Al-Masri M, Krishnamurthy M, Li J, Fellows GF, Dong HH, Goodyer CG, Wang R. Effect of forkhead box O1 (FOXO1) on beta cell development in the human fetal pancreas. Diabetologia. 2010;53:699–711. - PubMed
-
- Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab. 2007;3:758–768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

