A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread
- PMID: 22980983
- PMCID: PMC3514884
- DOI: 10.1016/j.cell.2012.07.021
A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread
Abstract
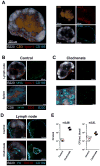
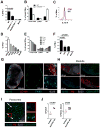
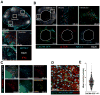
The lymphatic network that transports interstitial fluid and antigens to lymph nodes constitutes a conduit system that can be hijacked by invading pathogens to achieve systemic spread unless dissemination is blocked in the lymph node itself. Here, we show that a network of diverse lymphoid cells (natural killer cells, γδ T cells, natural killer T cells, and innate-like CD8+ T cells) are spatially prepositioned close to lymphatic sinus-lining sentinel macrophages where they can rapidly and efficiently receive inflammasome-generated IL-18 and additional cytokine signals from the pathogen-sensing phagocytes. This leads to rapid IFNγ secretion by the strategically positioned innate lymphocytes, fostering antimicrobial resistance in the macrophage population. Interference with this innate immune response loop allows systemic spread of lymph-borne bacteria. These findings extend our understanding of the functional significance of cellular positioning and local intercellular communication within lymph nodes while emphasizing the role of these organs as highly active locations of innate host defense.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures

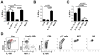


References
-
- Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. - PubMed
-
- Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii S, Tanaka M. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34:85–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

