Long-distance growth and connectivity of neural stem cells after severe spinal cord injury
- PMID: 22980985
- PMCID: PMC3445432
- DOI: 10.1016/j.cell.2012.08.020
Long-distance growth and connectivity of neural stem cells after severe spinal cord injury
Abstract
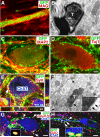
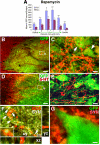
Neural stem cells (NSCs) expressing GFP were embedded into fibrin matrices containing growth factor cocktails and grafted to sites of severe spinal cord injury. Grafted cells differentiated into multiple cellular phenotypes, including neurons, which extended large numbers of axons over remarkable distances. Extending axons formed abundant synapses with host cells. Axonal growth was partially dependent on mammalian target of rapamycin (mTOR), but not Nogo signaling. Grafted neurons supported formation of electrophysiological relays across sites of complete spinal transection, resulting in functional recovery. Two human stem cell lines (566RSC and HUES7) embedded in growth-factor-containing fibrin exhibited similar growth, and 566RSC cells supported functional recovery. Thus, properties intrinsic to early-stage neurons can overcome the inhibitory milieu of the injured adult spinal cord to mount remarkable axonal growth, resulting in formation of new relay circuits that significantly improve function. These therapeutic properties extend across stem cell sources and species.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Building bridges for spinal cord repair.Cell. 2012 Sep 14;150(6):1105-6. doi: 10.1016/j.cell.2012.08.025. Cell. 2012. PMID: 22980974
-
Long-distance migration and colonization of transplanted neural stem cells.Cell. 2014 Jan 30;156(3):385-7. doi: 10.1016/j.cell.2014.01.017. Cell. 2014. PMID: 24485444 No abstract available.
References
-
- Baska KM, Manandhar G, Feng D, Agca Y, Tengowski MW, Sutovsky M, Yi YJ, Sutovsky P. Mechanism of extracellular ubiquitination in the mammalian epididymis. J Cell Physiol. 2008;215:684–696. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. - PubMed
-
- Boulis NM, Federici T, Glass JD, Lunn JS, Sakowski SA, Feldman EL. Translational stem cell therapy for amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;8:172–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

