Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures
- PMID: 22981233
- PMCID: PMC3462245
- DOI: 10.1016/j.celrep.2012.08.002
Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures
Abstract
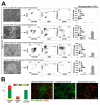
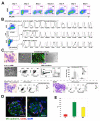
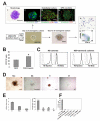
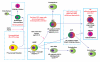
Hemogenic endothelium (HE) has been recognized as a source of hematopoietic stem cells (HSCs) in the embryo. Access to human HE progenitors (HEPs) is essential for enabling the investigation of the molecular determinants of HSC specification. Here, we show that HEPs capable of generating definitive hematopoietic cells can be obtained from human pluripotent stem cells (hPSCs) and identified precisely by a VE-cadherin(+)CD73(-)CD235a/CD43(-) phenotype. This phenotype discriminates true HEPs from VE-cadherin(+)CD73(+) non-HEPs and VE-cadherin(+)CD235a(+)CD41a(-) early hematopoietic cells with endothelial and FGF2-dependent hematopoietic colony-forming potential. We found that HEPs arise at the post-primitive-streak stage of differentiation directly from VE-cadherin-negative KDR(bright)APLNR(+)PDGFRα(low/-) hematovascular mesodermal precursors (HVMPs). In contrast, hemangioblasts, which are capable of forming endothelium and primitive blood cells, originate from more immature APLNR(+)PDGFRα(+) mesoderm. The demarcation of HEPs and HVMPs provides a platform for modeling blood development from endothelium with a goal of facilitating the generation of HSCs from hPSCs.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. - PubMed
-
- Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W, Dejana E. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood. 1996;87:630–641. - PubMed
-
- Carpenter L, Malladi R, Yang CT, French A, Pilkington KJ, Forsey RW, Sloane-Stanley J, Silk KM, Davies TJ, Fairchild PJ, et al. Human induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood. 2011;117:4008–4011. - PubMed
-
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

