An emerging link in stem cell mobilization between activation of the complement cascade and the chemotactic gradient of sphingosine-1-phosphate
- PMID: 22981511
- PMCID: PMC5539336
- DOI: 10.1016/j.prostaglandins.2012.07.003
An emerging link in stem cell mobilization between activation of the complement cascade and the chemotactic gradient of sphingosine-1-phosphate
Abstract
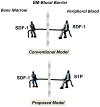
Under steady-state conditions, hematopoietic stem/progenitor cells (HSPCs) egress from bone marrow (BM) and enter peripheral blood (PB) where they circulate at low levels. Their number in PB, however, increases significantly in several stress situations related to infection, organ/tissue damage, or strenuous exercise. Pharmacologically mediated enforced egress of HSPCs from the BM microenvironment into PB is called "mobilization", and this phenomenon has been exploited in hematological transplantology as a means to obtain HSPCs for hematopoietic reconstitution. In this review we will present the accumulated evidence that innate immunity, including the complement cascade and the granulocyte/monocyte lineage, and the PB plasma level of the bioactive lipid sphingosine-1-phosphate (S1P) together orchestrate this evolutionarily conserved mechanism that directs trafficking of HSPCs.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Mikkola HKA, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–44. - PubMed
-
- Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004;103:1580–85. - PubMed
-
- Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, et al. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24:1667–75. - PubMed
-
- Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–92. - PubMed
-
- Doan PL, Chute JP. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2012;26:54–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

