Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection
- PMID: 22981788
- PMCID: PMC4284103
- DOI: 10.1016/j.jaci.2012.07.031
Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection
Abstract
Background: Respiratory viral infection, including respiratory syncytial virus (RSV) and rhinovirus, has been linked to respiratory disease in pediatric patients, including severe acute bronchiolitis and asthma exacerbation.
Objective: The study examined the role of the epithelial-derived cytokine thymic stromal lymphopoietin (TSLP) in the response to RSV infection.
Methods: Infection of human airway epithelial cells was used to examine TSLP induction after RSV infection. Air-liquid interface cultures from healthy children and children with asthma were also tested for TSLP production after infection. Finally, a mouse model was used to directly test the role of TSLP signaling in the response to RSV infection.
Results: Infection of airway epithelial cells with RSV led to the production of TSLP via activation of an innate signaling pathway that involved retinoic acid induced gene I, interferon promoter-stimulating factor 1, and nuclear factor-κB. Consistent with this observation, airway epithelial cells from asthmatic children a produced significantly greater levels of TSLP after RSV infection than cells from healthy children. In mouse models, RSV-induced TSLP expression was found to be critical for the development of immunopathology.
Conclusion: These findings suggest that RSV can use an innate antiviral signaling pathway to drive a potentially nonproductive immune response and has important implications for the role of TSLP in viral immune responses in general.
Copyright © 2012 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
Figures

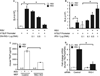
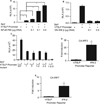



Comment in
-
Interferon regulatory factor 3 activation mediates viral stimulus-induced bronchial production of thymic stromal lymphopoietin.J Allergy Clin Immunol. 2013 Mar;131(3):926. doi: 10.1016/j.jaci.2012.11.034. Epub 2013 Jan 12. J Allergy Clin Immunol. 2013. PMID: 23321204 No abstract available.
-
Reply: To PMID 22981788.J Allergy Clin Immunol. 2013 Mar;131(3):926-7. doi: 10.1016/j.jaci.2012.11.035. J Allergy Clin Immunol. 2013. PMID: 23452904 No abstract available.
References
-
- Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. - PubMed
-
- Bueno SM, Gonzalez PA, Pacheco R, Leiva ED, Cautivo KM, Tobar HH, et al. Host immunity during RSV pathogenesis. Int Immunopharmacol. 2008;8:1320–1329. - PubMed
-
- Lukacs NW, Tekkanat KK, Berlin A, Hogaboam CM, Miller A, Evanoff HL, et al. Respiratory syncytial virus predisposes mice to augmented allergic airway responses via IL-13-mediated mechanisms. J Immunol. 2001;167:1060–1065. - PubMed
-
- Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL098067/HL/NHLBI NIH HHS/United States
- R21 HL102708/HL/NHLBI NIH HHS/United States
- R01 HL059178/HL/NHLBI NIH HHS/United States
- R56 AI060389/AI/NIAID NIH HHS/United States
- AI083019/AI/NIAID NIH HHS/United States
- AR056113/AR/NIAMS NIH HHS/United States
- AR055695/AR/NIAMS NIH HHS/United States
- R01 AR056113/AR/NIAMS NIH HHS/United States
- HL098067/HL/NHLBI NIH HHS/United States
- U19 AI083019/AI/NIAID NIH HHS/United States
- AI060389/AI/NIAID NIH HHS/United States
- R01 AR055695/AR/NIAMS NIH HHS/United States
- R01 AI060389/AI/NIAID NIH HHS/United States
- AI068731/AI/NIAID NIH HHS/United States
- R01 AI068731/AI/NIAID NIH HHS/United States
- HL059178/HL/NHLBI NIH HHS/United States
- HL102708/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

