Role of the retinal vascular endothelial cell in ocular disease
- PMID: 22982179
- PMCID: PMC3679193
- DOI: 10.1016/j.preteyeres.2012.08.004
Role of the retinal vascular endothelial cell in ocular disease
Abstract
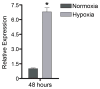
Retinal endothelial cells line the arborizing microvasculature that supplies and drains the neural retina. The anatomical and physiological characteristics of these endothelial cells are consistent with nutritional requirements and protection of a tissue critical to vision. On the one hand, the endothelium must ensure the supply of oxygen and other nutrients to the metabolically active retina, and allow access to circulating cells that maintain the vasculature or survey the retina for the presence of potential pathogens. On the other hand, the endothelium contributes to the blood-retinal barrier that protects the retina by excluding circulating molecular toxins, microorganisms, and pro-inflammatory leukocytes. Features required to fulfill these functions may also predispose to disease processes, such as retinal vascular leakage and neovascularization, and trafficking of microbes and inflammatory cells. Thus, the retinal endothelial cell is a key participant in retinal ischemic vasculopathies that include diabetic retinopathy and retinopathy of prematurity, and retinal inflammation or infection, as occurs in posterior uveitis. Using gene expression and proteomic profiling, it has been possible to explore the molecular phenotype of the human retinal endothelial cell and contribute to understanding of the pathogenesis of these diseases. In addition to providing support for the involvement of well-characterized endothelial molecules, profiling has the power to identify new players in retinal pathologies. Findings may have implications for the design of new biological therapies. Additional progress in this field is anticipated as other technologies, including epigenetic profiling methods, whole transcriptome shotgun sequencing, and metabolomics, are used to study the human retinal endothelial cell.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- Adamis AP, Shima DT, Yeo KT, Yeo TK, Brown LF, Berse B, D’Amore PA, Folkman J. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993;193:631–638. - PubMed
-
- Aguilar E, Dorrell MI, Friedlander D, Jacobson RA, Johnson A, Marchetti V, Moreno SK, Ritter MR, Friedlander M. Chapter 6. Ocular models of angiogenesis. Methods Enzymol. 2008;444:115–158. - PubMed
-
- Ahn JK, Yu HG, Chung H, Park YG. Intraocular cytokine environment in active Behcet uveitis. American journal of ophthalmology. 2006;142:429–434. - PubMed
-
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. The New England journal of medicine. 1994;331:1480–1487. - PubMed
-
- Aird WC. Mechanisms of endothelial cell heterogeneity in health and disease. Circ Res. 2006;98:159–162. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

