Endoplasmic reticulum stress induces the expression of COX-2 through activation of eIF2α, p38-MAPK and NF-κB in advanced glycation end products stimulated human chondrocytes
- PMID: 22982228
- PMCID: PMC4509732
- DOI: 10.1016/j.bbamcr.2012.08.021
Endoplasmic reticulum stress induces the expression of COX-2 through activation of eIF2α, p38-MAPK and NF-κB in advanced glycation end products stimulated human chondrocytes
Abstract
Introduction: During aging, advanced glycation end products (AGEs) accumulate in articular cartilage. In this study we determined whether AGEs induce endoplasmic reticulum (ER) stress and studied the ER stress-activated pathways that stimulate cyclooxygenase-2 (COX-2) expression in human chondrocytes.
Methods: Chondrocytes were stimulated with AGE-BSA. Gene expression was determined by quantitative PCR and protein expression was studied by immunoblotting. Studies to elucidate involved pathways were executed using siRNAs and specific inhibitors of eukaryotic initiation factor-2α (eIF2α), MAPKs and NF-κB.
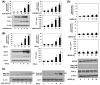
Results: AGE-BSA induced expression of GRP78 with concomitant increase in COX-2 expression was observed in human chondrocytes. In addition, expression of Bag-1, an ER stress marker was also increased by AGE-BSA. RAGE knockdown inhibited AGE-BSA-induced expression of GRP78 and COX-2. Treatment with eIF2α inhibitor or eIF2α knockdown inhibited AGE-BSA-induced expression of GRP78 and COX-2 with decreased PGE(2) production. Treatment with SB202190 inhibited AGE-BSA-induced expression of GRP78 and COX-2, while treatment with PD98051 inhibited AGE-BSA-induced GRP78 protein expression but had no effect on COX-2 protein expression. SP600125 had no effect on either GRP78 or COX-2 protein expression. Bay 11-7082 suppressed AGE-BSA-induced GRP78 and COX-2 expression. AGE-BSA-induced activation of NF-κB was inhibited by treatment with SB202190 and by eIF2α knockdown, but was not inhibited when chondrocytes were treated with SP600125 or PD98059.
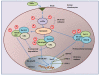
Conclusion: This study demonstrates that AGEs induce ER stress and stimulate the expression of COX-2 through eIF2α, p38-MAPK and NF-κB pathways in human chondrocytes. Our results provide important insights into cartilage degradation in osteoarthritis associated with latent ER stress.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures





References
-
- Yang L, Carlson SG, McBurney D, Horton WE., Jr. Multiple signals induce endoplasmic reticulum stress in both primary and immortalized chondrocytes resulting in loss of differentiation, impaired cell growth, and apoptosis. J. Biol. Chem. 2005;280:31156–31165. - PubMed
-
- Yang L, McBurney D, Tang SC, Carlson SG, Horton WE., Jr. A novel role for Bcl-2 associated-athanogene-1 (Bag-1) in regulation of the endoplasmic reticulum stress response in mammalian chondrocytes. J. Cell. Biochem. 2007;102:786–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

